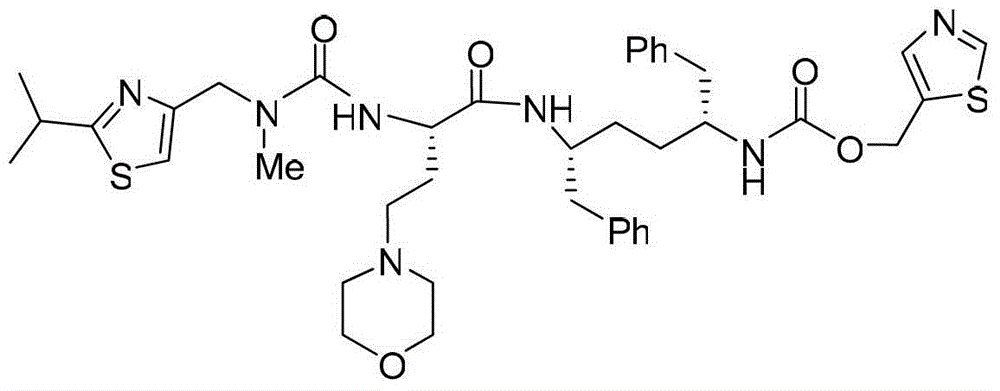
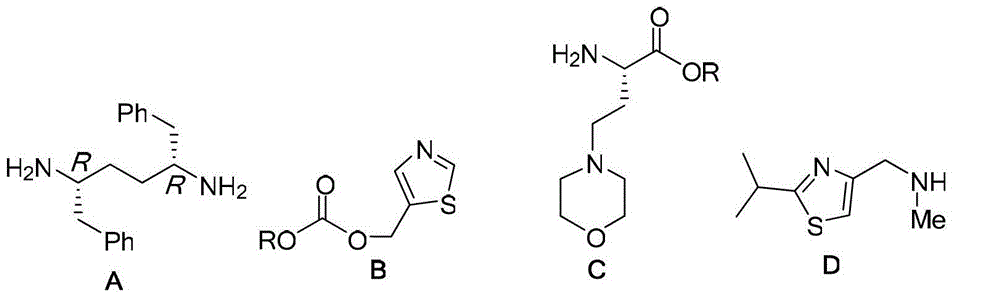
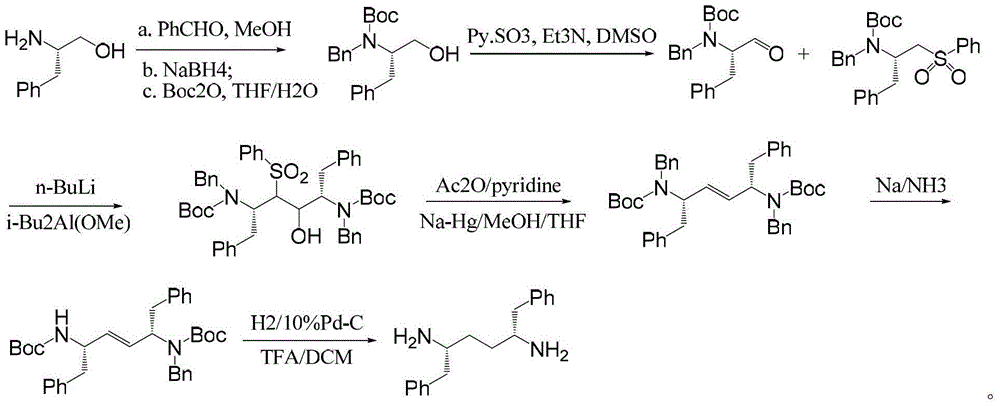
Intermediates for synthesizing (2R,5R)-1,6-diphenylhexyl-2,5-diamine and salts thereof, preparation method and applications of the intermediates
A technology of diphenylhexane and intermediates, applied in the field of organic synthesis, can solve the problems of being unsuitable for large-scale industrial production, harsh reaction conditions, difficult post-processing, etc., and achieve easy-to-operate post-processing, mild reaction conditions, and excellent preparation methods simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 (2S, 3S, 5S)-5-(tert-butoxycarbonylamino)-2-(dibenzylamino)-1,6-diphenyl-3-hexyl methylsulfonate (IIa) synthesis
[0041] The compound of formula I (10g, 17.7mmol) was dissolved in 50g of dichloromethane, triethylamine (1.88g, 18.6mmol) was added, cooled to 0-10°C, methanesulfonyl chloride (2.18g, 19.0mol) was added dropwise, React at room temperature until the compound of formula I disappears (TLC detection, n-hexane / ethyl acetate=5 / 1, V / V); cool to 0-10°C, wash the reaction solution with saturated sodium bicarbonate solution (50mL×3), separate liquid, concentrated under reduced pressure to obtain 10.4 g of the compound of formula IIa, the molar yield was 91%, and the HPLC purity was 98.1%.
[0042] MS(ESI)m / z:M+1=643.3;
[0043] 1 H NMR (CDCl 3 400MHz) δ1.42(m, 9H), 1.68(t, 2H), 2.80~2.92(m, 4H), 3.05(s, 3H), 3.21(s, 4H), 4.25(m, 1H), 4.93( m, 1H), 7.26-7.55 (m, 20H).
Embodiment 2
[0044] Example 2 (2S, 3S, 5S)-5-(tert-butoxycarbonylamino)-2-(dibenzylamino)-1,6-diphenyl-3-hexylethylsulfonate (IIb) synthesis
[0045] The compound of formula I (10g, 17.7mmol) was dissolved in 50g of tetrahydrofuran, diisopropylethylamine (2.4g, 18.6mmol) was added, cooled to 0-10°C, and ethanesulfonyl chloride (2.44g, 19.0 mol), react at room temperature until the compound of formula I disappears (TLC detection, n-hexane / ethyl acetate=5 / 1, V / V); cool to 0-10°C, and use saturated sodium bicarbonate solution (50mL×3) Washing, liquid separation, and concentration under reduced pressure gave 10.2 g of the compound of formula IIb with a molar yield of 88% and an HPLC purity of 96.9%.
[0046] MS(ESI)m / z:M+1=657.6;
[0047] 1 H NMR (CDCl 3 400MHz) δ1.29(t, 3H), 1.43(m, 9H), 1.69(t, 2H), 2.80~2.92(m, 4H), 3.08(s, 2H), 3.24(s, 4H), 4.23( m, 1H), 4.95 (m, 1H), 7.24-7.58 (m, 20H).
Embodiment 3
[0048]Example 3 (2S, 3S, 5S)-5-(tert-butoxycarbonylamino)-2-(dibenzylamino)-1,6-diphenyl-3-hexyl p-toluenesulfonate (IIc) synthesis
[0049] Dissolve the compound of formula I (10g, 17.7mmol) in 100g toluene, add a 20% aqueous solution made of sodium carbonate (2.0g, 19mmol), cool to -5~0°C, add p-toluenesulfonyl chloride (3.8g, 20.0mol) in 10g of toluene solution, react at room temperature until the compound of formula I disappears (TLC detection, n-hexane / ethyl acetate=5 / 1, V / V); cool to 10-15°C, and use saturated sodium bicarbonate solution for the reaction solution (50mL×3) washed, separated, and concentrated the organic phase under reduced pressure to obtain 11.4g of the compound of formula IIc, with a molar yield of 90% and an HPLC purity of 95.8%.
[0050] MS(ESI)m / z:M+1=720.0;
[0051] 1 H NMR (CDCl 3 400MHz) δ1.43(m, 9H), 2.80~2.92(m, 4H), 3.08(s, 2H), 3.24(s, 4H), 4.23(m, 1H), 4.95(m, 1H), 7.22~ 7.61 (m, 24H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com