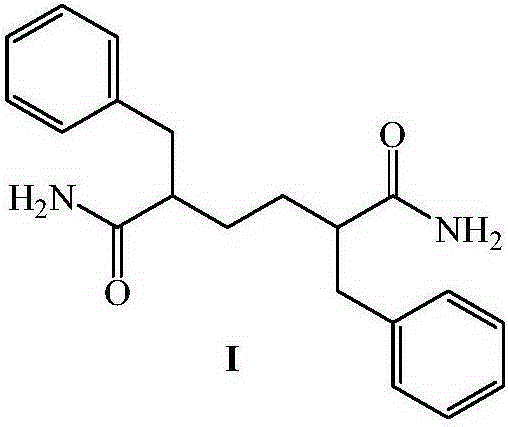
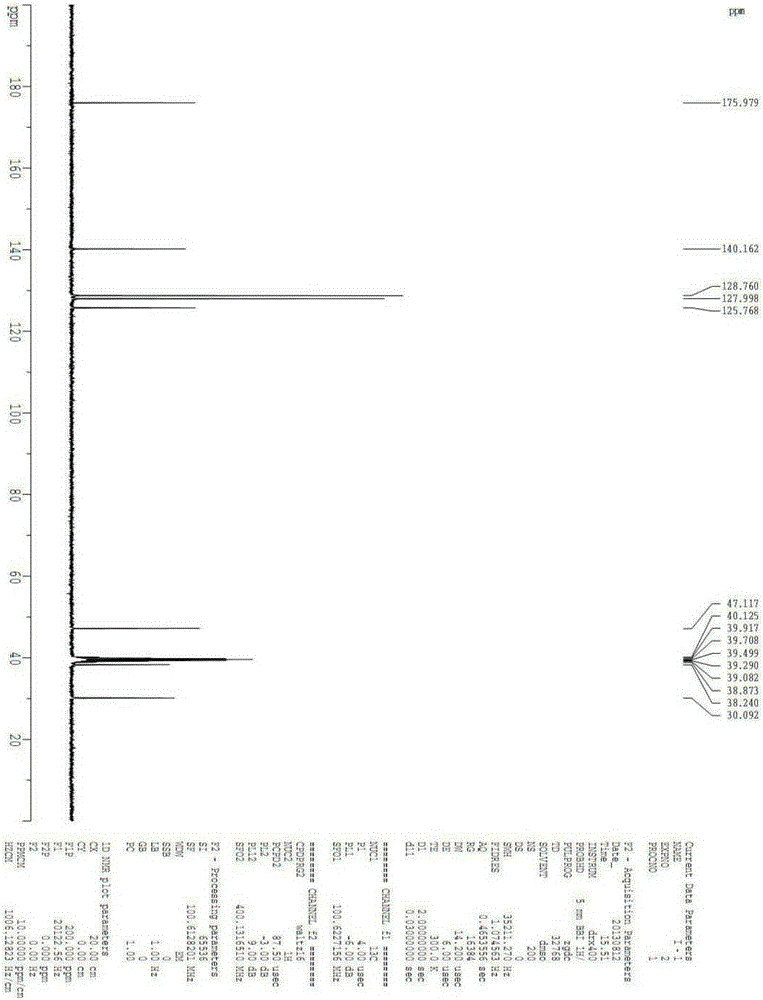
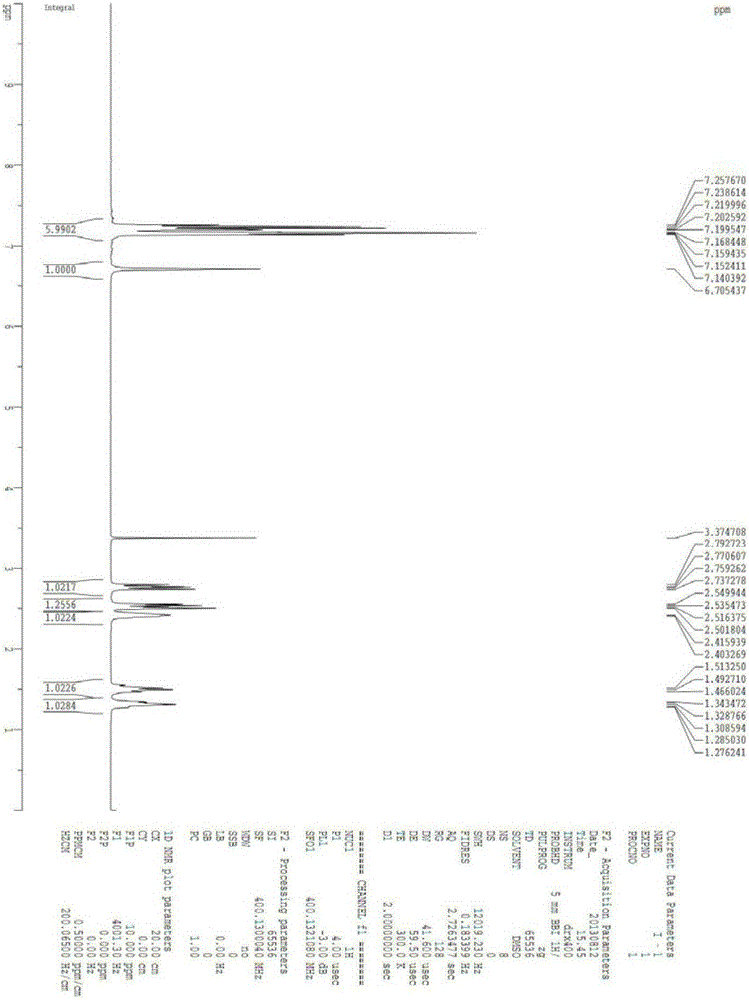
Intermediate for the synthesis of anti-AIDS drug enhancer cobicistat
An anti-AIDS and intermediate technology, which is applied in the field of new intermediate compounds of cobicistat, an anti-AIDS drug enhancer, can solve the problems of harsh reaction conditions, huge energy consumption, and many reaction steps, and meet high quality requirements and high product purity , the effect of low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1, preparation compound I
[0063]
[0064] In a 500ml four-necked flask, 60g of compound II and 200ml of thionyl chloride were put in, and the reaction was refluxed for 2 hours. The reaction solution was dissolved and clarified. Samples were tested by HPLC until the basic reaction of the raw materials was complete, and the reaction was stopped. The reaction solution was kept below 50°C, and excess thionyl chloride was distilled off under reduced pressure, and the residue was set aside for use. In another 1000ml four-necked bottle, add 250ml of ammonia water with a concentration of 15%, stir, cool to 10-15°C, add the above-mentioned concentrated residue dropwise, after about 1 hour, a large amount of white solid is formed, and the temperature of the reaction solution is raised to Continue to react at 15-20°C for 2h, filter, wash the filter cake with 200ml of water, and dry at 70°C to obtain 52.5g of white solid, yield 87.5%, melting point, 204.8-211.8°C, N...
Embodiment 2
[0065] Embodiment 2, preparation compound III
[0066]
[0067] In a 500ml four-necked bottle, add 20g of sodium hydroxide, 150ml of water, 100ml of industrial grade sodium hypochlorite solution (approximately 9.5% available chlorine content), stir, cool to about 10°C, and start to add 52.5g of compound I several times. Rise to 2-5°C, remove the cooling, naturally warm to room temperature, and keep warm for 1.5h, take samples, and use TLC to detect until the raw materials are completely reacted. In another 1000ml four-necked bottle, add 150ml of water, heat to 75-78°C, add the above reaction solution dropwise in about 1 hour, after the drop is complete, continue the heat preservation reaction at 75-78°C for 3-4 hours, and then stop heating , cooled to room temperature, extracted the product with dichloromethane, and concentrated to dryness to obtain 34 g of oil.
Embodiment 3
[0068] Embodiment 3, preparation compound III-1
[0069]
[0070] In a 500ml reaction bottle, add 34g of the condensate obtained in the previous step, 150ml of methanol, stir to dissolve and clarify, add 29.5g of D-camphorsulfonic acid at room temperature, heat the reaction solution until it is dissolved and clear, then cool, and a white solid is continuously precipitated. After 4h, slowly cool the reaction solution to room temperature, continue to stir and crystallize for 2h, filter to obtain 15.7g of white solid,
[0071] Put 15.7g of the white solid obtained in the previous step, 72ml of dichloromethane, and 36ml of water into a 200ml four-necked bottle, stir and dissolve until clear, adjust the pH value to about 10 with aqueous sodium hydroxide solution, continue stirring for 10min, and let it stand for stratification. The aqueous layer was extracted with 20ml*2 dichloromethane, the combined organic layers were washed once with 20ml of water, the organic layer was dried...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com