A real-time assay method and kit for methyltransferase activity
A methyltransferase and real-time assay technology, which is applied to measuring devices, instruments, scientific instruments, etc., can solve problems such as product instability, detection value deviation, and many influencing factors, and achieve accurate MT activity determination and accurate and timely results. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
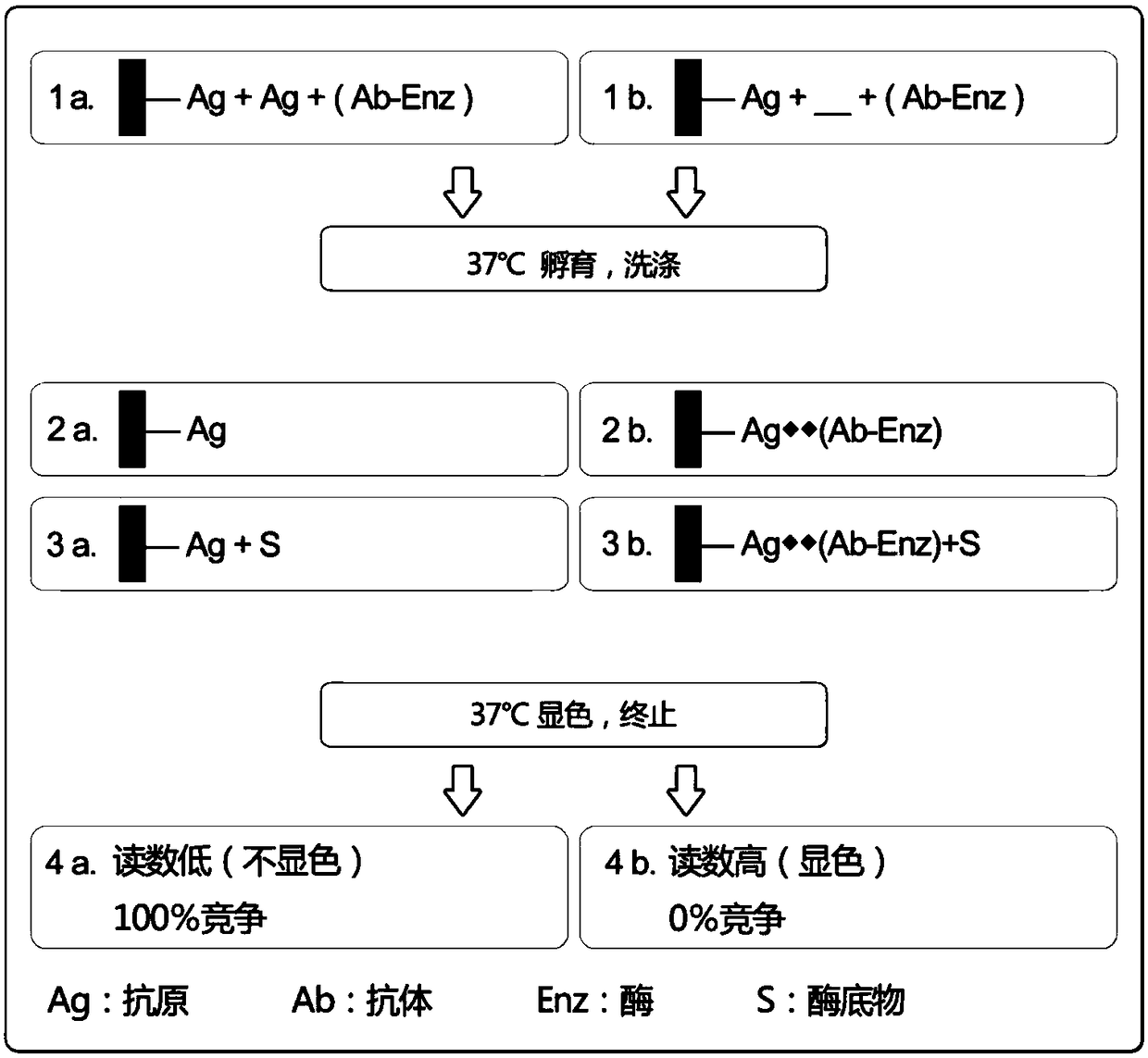
[0066] Example 1 Anti-SAH antibody cross-reacts with homocysteine methyltransferase substrate SAM and Hcy
[0067] Experimental Materials
[0068] HRP enzyme-labeled anti-SAH antibody: Arthus Biosystems, MAH00301; bovine serum albumin BSA-SAH: Arthus Biosystems, ACT00301; S-adenosyl homocysteine (SAH) standard: Arthus Biosystems, AST00301;
[0069] S-adenosylmethionine: Sigma, A2408,; Homocysteine methyltransferase (HMT): Abt-P-005 of Beijing Aibison Biotechnology Co., Ltd.; BSA, Tris-Cl, NaCl, NaH 2 PO 4 , Na 2 HPO 4 , Na 2 HPO 4 .12H 2 O: Beijing Huamei Jiacheng Biotechnology Co., Ltd.; KCl: Tianjin Damao Chemical Reagent Factory; Homocysteine (Hcy), ProClin 300: Sigma; TMB Chromogenic Solution: Huzhou Yingchuang Biotechnology Co., Ltd.; Sulfuric acid: Hunan Kangdu Pharmaceutical Co., Ltd.; 96-well enzyme-linked plate: American Corning high-adsorption enzyme-linked plate.
[0070] Reagent preparation:
[0071] According to the initial experiment, the BSA-SAH c...
Embodiment 2
[0080] Embodiment 2: In vitro determination of the most suitable buffer system for HMT activity
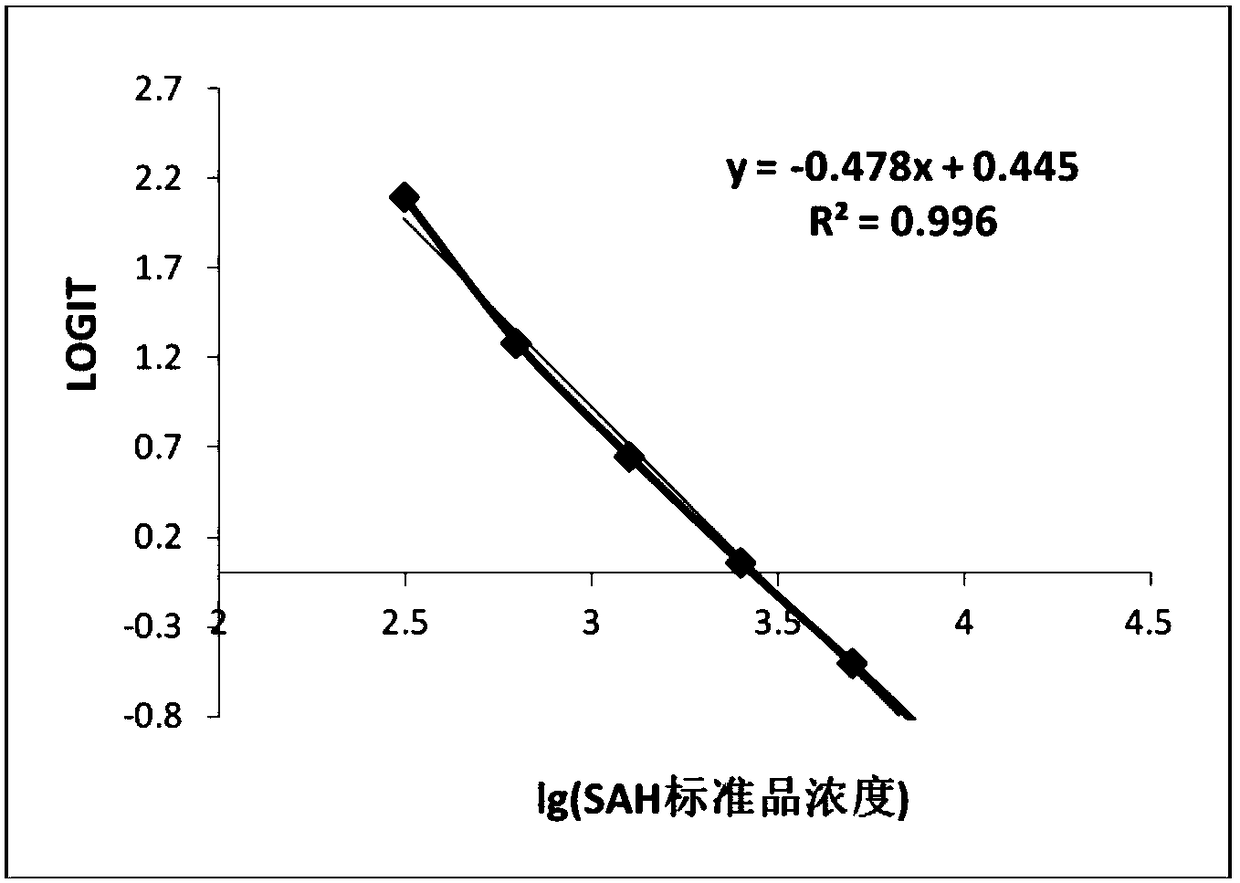
[0081] Take the ELISA plates coated with BSA-SAH antigen, and use 20mM Tris buffer of pH7.79, pH8.04, pH8.23, pH8.39 and pH7.0, pH7.4, pH7.8, pH8.0 respectively 100mM PB buffer was used to prepare the HMT system reaction solution containing 20uM SAM, 20uM Hcy and 1ug / ml HMT, the reaction solution was 50ul per well, and the HRP-labeled antibody was 50ul per well. React at 37°C for 20min, add 100ul TMB to each well after washing the plate, develop color at 37°C for 15min, add 50ul of stop solution and read. Among them, SAH standard curve uses 0, 0.3125, 0.625, 1.25, 2.5, 5, 10uM standard products, and is prepared with pH 7.8 PB buffer and pH 8.25 Tris buffer respectively. The detected curves were fitted, and the production of SAH in each experimental group was calculated respectively. The results are shown in the table below:
[0082] Table 3 Comparison of SAH formation catalyzed...
Embodiment 3
[0094] Embodiment 3: Enzyme concentration and substrate concentration of measuring HMT in vitro
[0095] Take the ELSIA plate coated with BSA-SAH antigen, prepare SAH standard 0, 0.3125, 0.625, 1.25, 2.5, 5, 10uM and 4uM quality control with enzyme reaction buffer pH7.8. And prepare different concentrations of SAM, Hcy and HMT enzyme formulations. Standards and enzyme reactions are 50ul per well, and HRP-labeled antibodies are 50ul per well. The reaction time at 37°C was 20min, 30min, and 40min. After washing the plate, 100ul TMB was allowed to develop color at 37°C for 15min, and read after 50ul of stop solution.
[0096] Table 6 Comparison of SAH formation catalyzed by HMT under different substrate concentrations and HMT concentrations
[0097]
[0098] According to the results in the table above, an increase in the concentration of SAM will cause an increase in the background value, which is caused by the cross-reaction between SAM and anti-SAH monoclonal antibody. At...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com