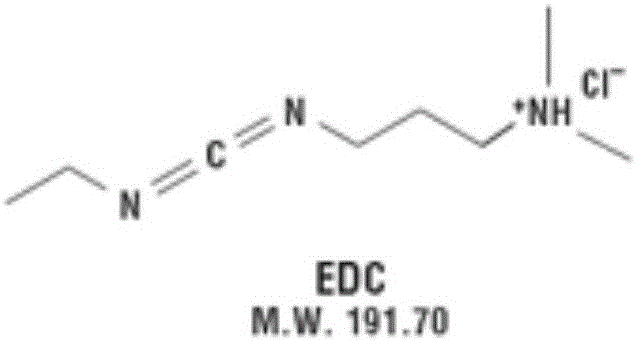
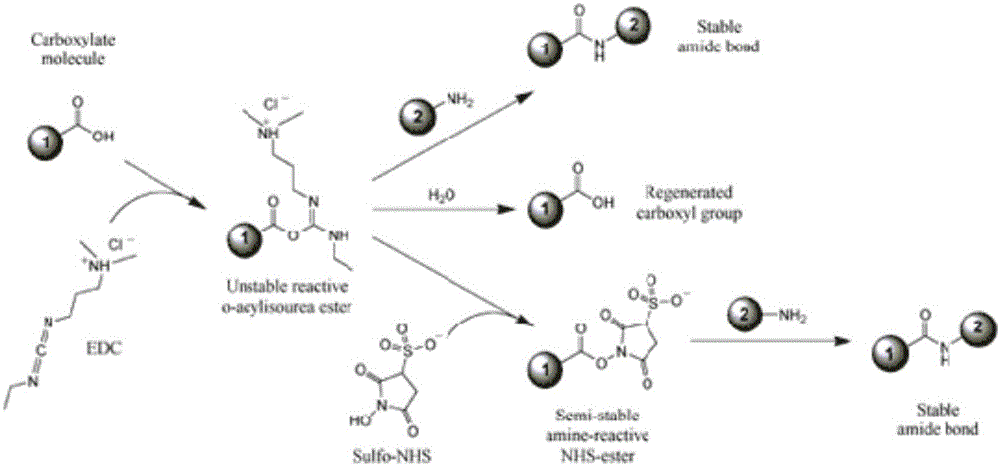
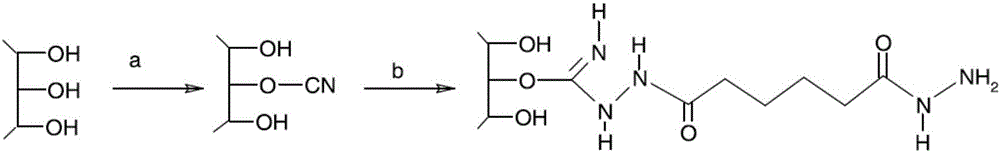
Preparation method of pneumococcal conjugate combination vaccine
A pneumococcal and combined vaccine technology, applied in vaccines, multivalent vaccines, antibacterial drugs, etc., can solve problems such as unsatisfactory effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1: Pneumococcus bacterial culture
[0057] After the strains are turned on, transfer them to Columbia blood agar medium, culture them at 37°C, recover and grow colonies, transfer them to a small amount of improved pancreatic soybean liquid medium, cultivate them for 8-12 hours, and transfer them to the second-generation improved pancreatic soybean liquid medium. Soybean strains are cultivated in liquid medium, and then transferred to a fermenter containing 35000ml of liquid medium for cultivation, during which the pH value is maintained at about 7.0, and the bacteria are inactivated at the end of the logarithmic growth phase.
Embodiment 2
[0058] Example 2: Purification of pneumococcal capsular polysaccharide
[0059] After the culture supernatant was centrifuged, concentrated by 100K membrane ultrafiltration, the buffer was replaced with 0.01mol / L phosphate buffer, the pH value was adjusted to 6.8-7.4, and cetyltrimethylammonium bromide was added at a final concentration of 1%. (CTAB), stir well and leave overnight. Collect the precipitate by centrifugation (collect supernatant for 7F and 14 types), add 0.25mol / L NaCl solution to dissociate CTAB (use 0.35mol / L NaCl solution for type 3), add 0.5% NaI, stir well, and collect supernatant by centrifugation , with ultrafiltration membrane ultrafiltration dialysis. After hydroxyapatite chromatography, the target polysaccharide is collected, precipitated with 75% ethanol, washed with ethanol and acetone, and dried to obtain purified polysaccharide.
Embodiment 3
[0060] Example 3: Test results of purified polysaccharides
[0061] The 24 pneumococcal capsular polysaccharides were purified separately, with 3 batches for each type, and the quality inspection results of each batch are shown in Table 3:
[0062]Table 3. Quality detection results of pneumococcal capsular polysaccharide
[0063]
[0064]
[0065]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com