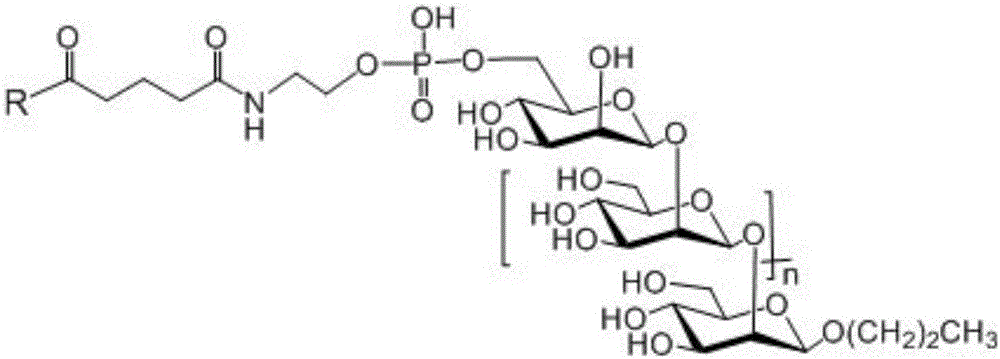
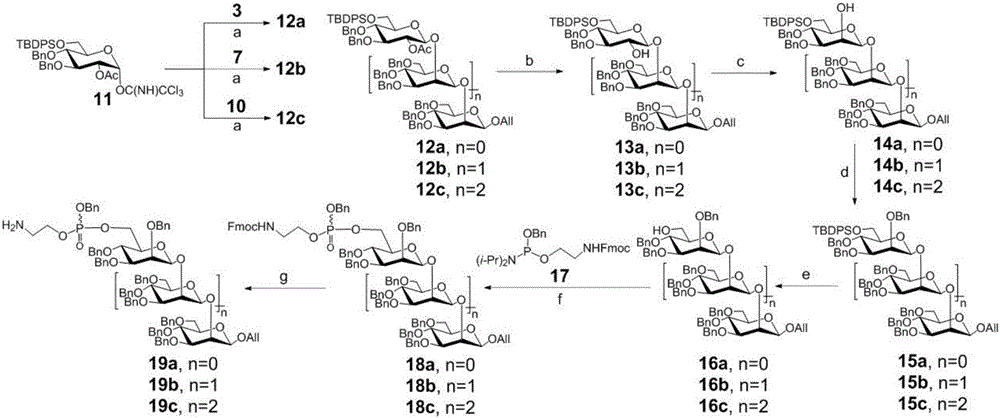
Beta-1,2-D-oligomeric mannoprotein conjugates and preparation method and application thereof
A technology of mannoproteins and conjugates, which is applied in the field of β-1,2-D-oligomannose protein conjugates and its preparation, can solve problems such as limited immune response, achieve strong application value, and prevent leukemia. Effects of candida infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
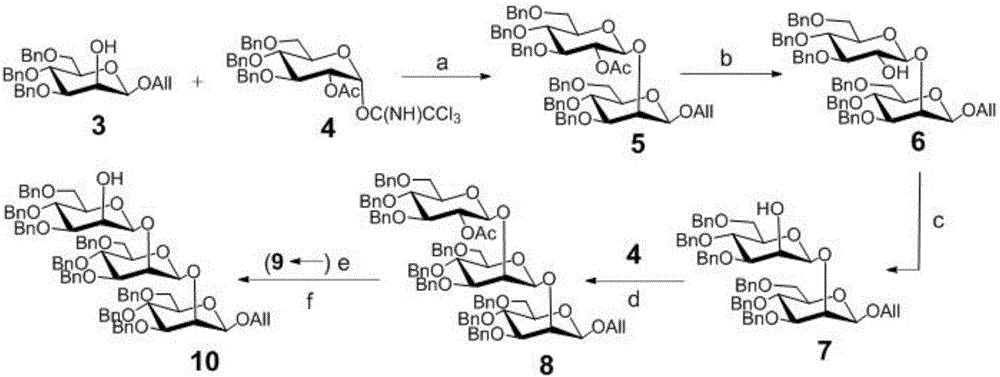
[0056] The synthesis of embodiment 1 compound 5
[0057] Monosaccharide acceptor 3 (1.23g, 2.5mmol) (J.Org.Chem.2001, 66, 8411.), donor 4 (1.95g, 3.0mmol) (J.Carbohydr.Chem.1994, 13, 421.) and activated Molecular sieves (1.0 g) were placed in a 100 mL round bottom flask and dried under vacuum for half an hour, then the mixture was dissolved in 50 mL of anhydrous dichloromethane. The suspension was stirred at room temperature under argon protection for half an hour, then cooled to -40°C, and then trimethylsilyl triflate (35.0 μL, 0.18 mmol) was slowly added dropwise. After half an hour, the reaction solution was neutralized and quenched with triethylamine and filtered with diatomaceous earth. After concentration, the initial product obtained was subjected to silica gel column chromatography (n-hexane / ethyl acetate, 12:1, v / v) to obtain a white foamy solid (2.23g, 92.4%).
[0058] 1 HNMR (400MHz, CDCl 3 )δ7.40-7.19(m,30H,Ar),5.88(m,1H,OCH 2 C H =CH 2 ),5.37(m,1H,OCH 2 ...
Embodiment 2
[0059] The synthesis of embodiment 2 compound 6
[0060] Compound 5 (1.85g, 2.0mmol) was dissolved in 100mL of anhydrous methanol, sodium methoxide (11.0mg, 0.2mmol) was added and stirred at room temperature overnight, after the reaction was completed, it was added to ion exchange resin IR 120 (H+form) And, the resin was removed by filtration, and the initial product obtained after concentration was subjected to silica gel column chromatography (n-hexane / ethyl acetate, 4:1, v / v) to obtain a white foamy solid (1.74g, 94.2%).
[0061] 1 H NMR (400MHz, CDCl 3 )δ7.55-7.23(m,30H,Ar),6.05-5.93(m,1H,OCH 2 C H =CH 2 ),5.40(m,1H,OCH 2 CH=C H 2 ),5.29(m,1H,OCH 2 CH=C H 2 ), 5.17 (dd, J=11.2Hz, 1H, OCH 2 Ph),5.05-4.93(m,4H,OCH 2 Ph), 4.90 (d, J=11.2Hz, 1H, OCH 2 Ph), 4.81(d, J=7.6Hz, 1H, H-1'), 4.72(d, J=12.0Hz, 1H, OCH 2 Ph),4.67-4.46(m,10H,OCH 2 Ph,O CH 2 CH=CH 2 ,H-1), 4.36(d,J=3.2Hz,1H,H-2),4.17-4.10(m,1H,O CH 2 CH=CH 2 ),4.01(t,J=9.6Hz,1H,H-4),3.88-3.71(m,6H,H...
Embodiment 3
[0062] The synthesis of embodiment 3 compound 7
[0063] Compound 6 (1.38 g, 1.5 mmol) was placed in a 100 mL round bottom flask, and then dimethyl sulfoxide (22.0 mL) and acetic anhydride (11.0 mL) were added. The reaction solution was stirred at room temperature for 18 hours, extracted with ethyl acetate, washed with saturated sodium carbonate and brine respectively, then dried with anhydrous sodium sulfate, filtered and concentrated to obtain the initial product, which was spun twice with toluene. The residue was dissolved in 40 mL of anhydrous tetrahydrofuran and cooled to -78°C, and L-Selectride (1 M THF, 7.5mL) and stirred for fifteen minutes, then removed the cooling device and placed at room temperature to continue stirring for fifteen minutes, quenched the reaction with methanol and diluted with 50mL dichloromethane, and the solutions were diluted with hydrogen peroxide solution (5%, 30mL), sodium hydroxide solution (1 M , 30mL), sodium thiosulfate solution (5%, 30m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com