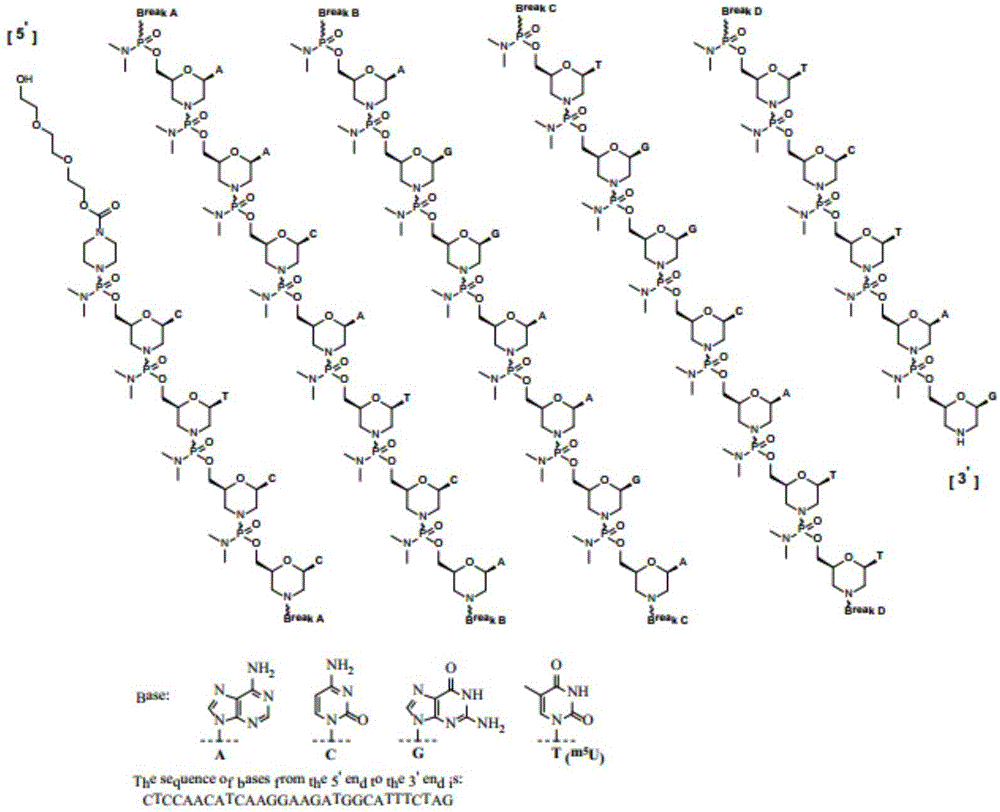
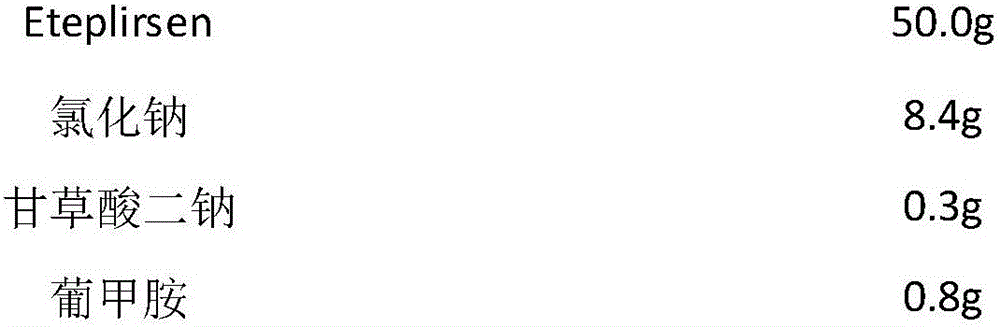Medicinal preparation suitable for Duchenne muscular dystrophy and preparation method thereof
A Duchenne muscle nutrition and pharmaceutical preparation technology, which is applied in the field of medicine, can solve the problems of insoluble particles becoming larger, insoluble particles and potency increasing, and potency decreasing, and achieve the effects of stable quality, improved safety, and convenient use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] prescription
[0035]
[0036]
[0037] Preparation Process
[0038] a. Ampoule treatment: wash the ampoule through an ultrasonic bottle washing machine, sterilize and dry it at 290°C in a tunnel type sterilization dryer, and transfer it to the filling room for standby;
[0039] B, preparation: 1. Eteplirsen and disodium glycyrrhizinate of the formula amount are weighed and dissolved in water for injection accounting for 30% of the total volume, and stirred and mixed for 15 minutes;
[0040] 2. Add water for injection to 1 Chinese medicinal liquid to 85% of the total volume, stir and mix evenly, add the prescribed amount of meglumine, stir the medicinal liquid to a colorless and clear liquid, and adjust the pH value with a pH regulator;
[0041] 3. Dilute to the total volume with water for injection, adjust the pH value with a pH regulator again, take a sample to test the intermediate product solution, and prepare for potting after passing the test.
[0042] c. ...
Embodiment 2
[0046] prescription
[0047]
[0048] Preparation process: with embodiment 1
Embodiment 3
[0050] prescription
[0051]
[0052]
[0053] Preparation process: with embodiment 1
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com