A combined vaccine for the prevention of Staphylococcus aureus infection and tetanus
A staphylococcus, combined vaccine technology, applied in the fields of genetic engineering and immunology, can solve problems such as affecting the stability of the vaccine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1. Preparation of recombinant Staphylococcus aureus truncated SasA protein
[0027] For obtaining the recombinant Staphylococcus aureus truncated SasA protein, refer to Chinese patent application CN102268444A. The disclosure of CN102268444A is incorporated into the description of the present invention by reference. The preparation method is briefly described below.
[0028] 1. According to the reported sequence of Staphylococcus Aureus MASA252 strain (GenBank: BX571856.1), design primers to amplify the sequence of 142-999bp as the target fragment, and its amino acid sequence is shown in SEQ ID NO.2, which is the first part of the full-length protein 48-333 amino acid segment. NdeI and XhoI restriction sites were added to both ends of the SasA sequence, and the stop codon was removed, and a sequence encoding 6 histidines was introduced at the 3' end.
[0029]2. After the amplified SasA gene was digested with NdeI and XhoI, it was connected to the expression vect...
Embodiment 2
[0033] Embodiment 2. Preparation of recombinant tetanus neurotoxin C fragment (TeNT-Hc)
[0034] For obtaining the recombinant TeNT-Hc protein, refer to Chinese patent CN101880675B. The present invention incorporates the disclosure of CN101880675B into the description of the present invention in the form of reference, and the preparation method is briefly described below.
[0035] 1. According to the sequencing results (GeneBank sequence number: AF154828) of 64008 virulent strains of Clostridium tetani C.Tetani in China, the Tet-Hc sequence of 451Aa was analyzed and optimized, and the optimized sequence was shown in SEQ ID NO.3. Whole Gene Synthesis.
[0036] 2. Construction of Tetanus Toxin Subunit Vaccine Hc Expression Vector
[0037] After the optimized synthetic Tet-Hc gene was digested with EcoRI and XhoI, it was connected to the expression vector pET32a(+) which was also digested with EcoRI and XhoI, transformed into Escherichia coli competent cell DH5α, and cultured ov...
Embodiment 3
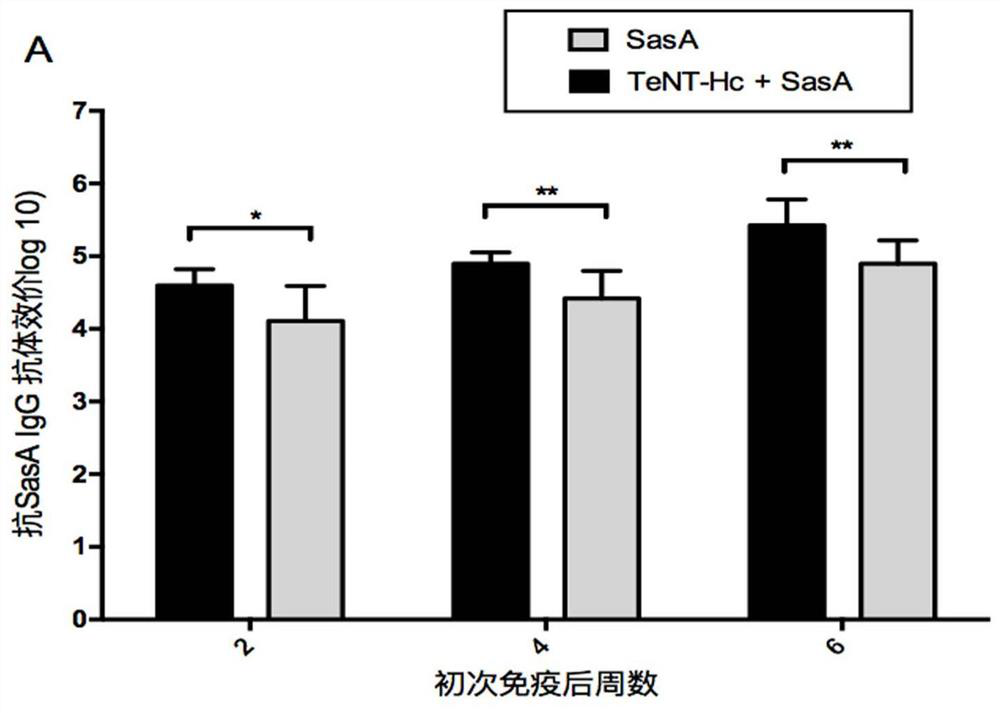
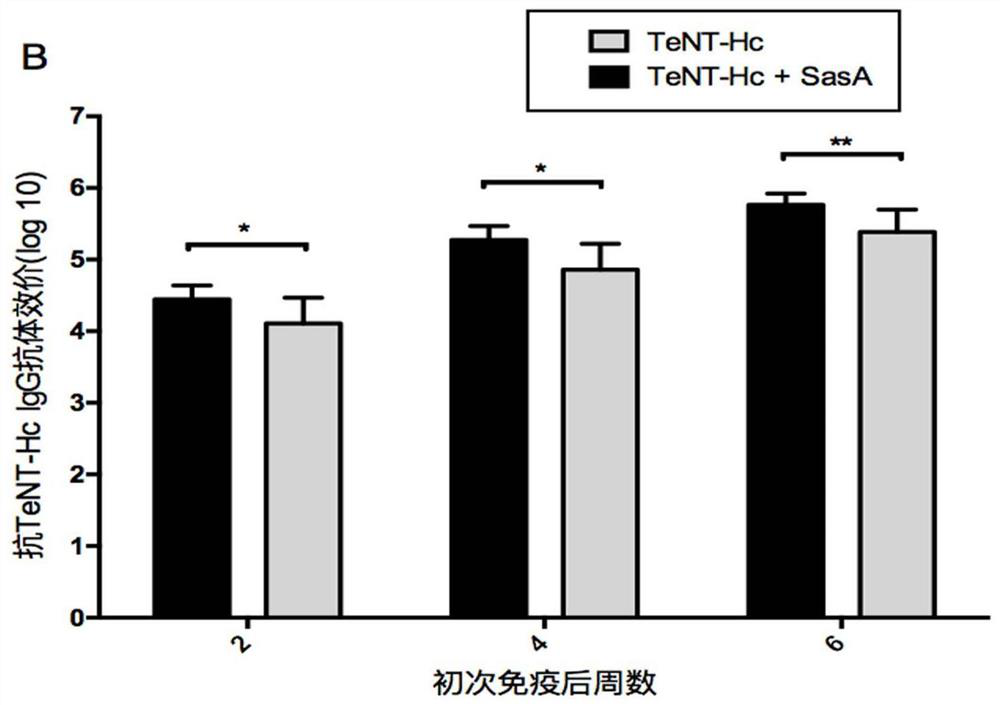
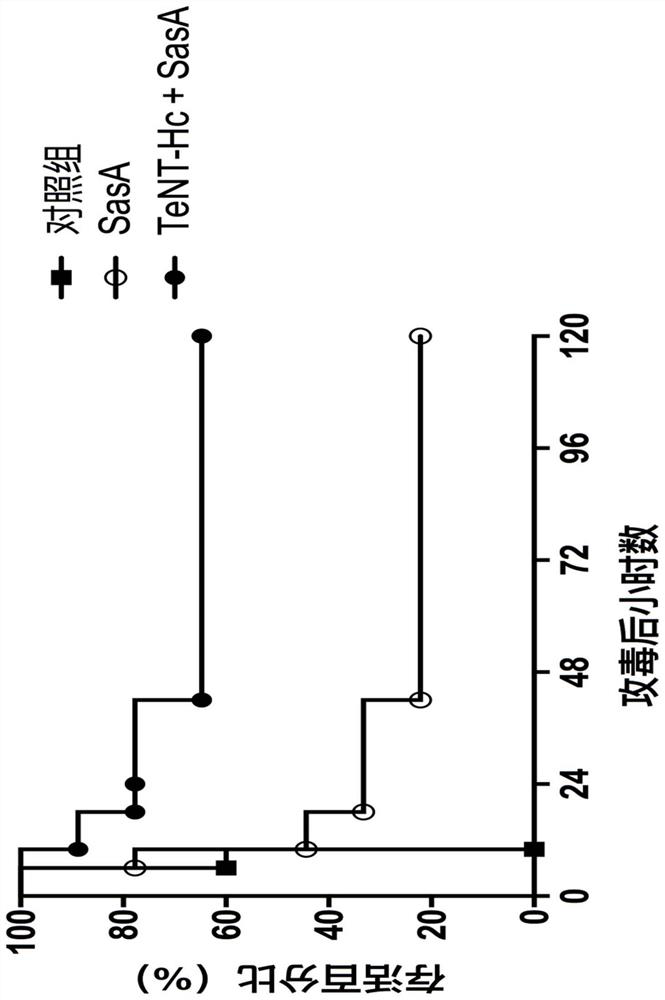
[0042] Example 3. Immunogenicity Research of SasA and TeNT-Hc Combined Vaccine
[0043] The following antigens were adsorbed with 0.75 mg aluminum hydroxide adjuvant: 10 μgrSasA, 10 μg TeNT-Hc, 10 μgrSasA+10 μg TeNT-Hc. 0.75 mg aluminum hydroxide adjuvant with no antigen adsorbed was used as negative control. At 0, 2 and 4 weeks, the above four groups of antigens were immunized with female BALB / c mice (aged 6-8 weeks) by intraperitoneal injection, 10 mice in each group. Blood samples were drawn from the tail vein and serum was extracted at 2, 4 and 6 weeks.
[0044] Serum antibody titers of antigen-specific IgG were analyzed by enzyme-linked immunosorbent assay (ELISA). 2 μg / ml of rSasA or TeNT-Hc was coated with 96-well enzyme-linked plate overnight at 4°C, and the coating buffer was 50 mM carbonate buffer (pH9.6). Blocking was incubated at 37° C. for 1 hour with PBS containing 2% (w / v) bovine serum albumin. The serially diluted serum was added to the enzyme-linked plate,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com