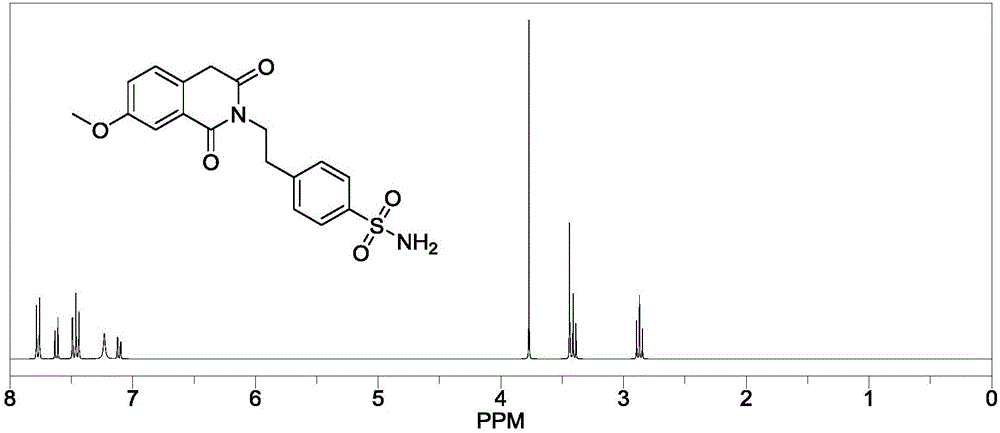
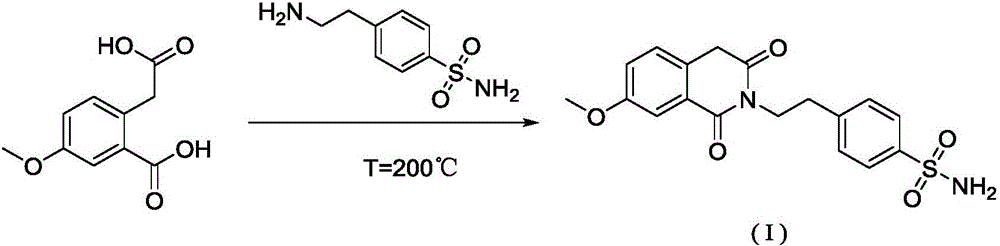
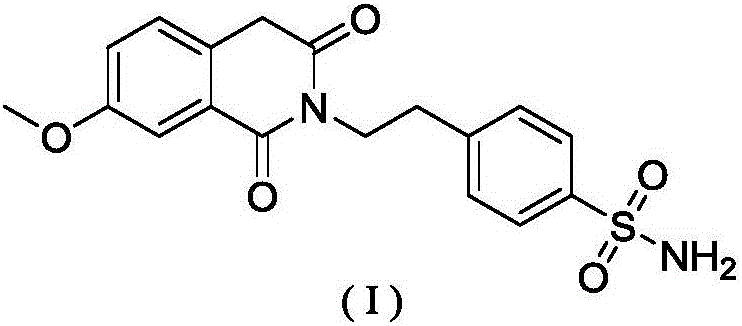
Method for preparing gliquidone intermediate
A technology of glipaquinone and intermediates, applied in the field of medicinal chemistry synthesis, can solve the problems of safety, hidden dangers of operation, high reaction temperature, harsh heating conditions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] S1: Add 50.00g of acrylic acid and 58.88g of thionyl chloride to a 500mL three-necked flask in turn, start stirring, turn on heating, raise the temperature to reflux temperature of 79±1°C, and continue the reflux reaction for 2 hours; after reflux for 2 hours, cool down slightly until no more reflux , install the distillation device, heat up and evaporate the remaining thionyl chloride, and after 30 minutes, remove the distillation device and change to vacuum distillation to distill the remaining traces of thionyl chloride in the three-necked flask until a small amount of white flocs appear on the condenser. until the solid is formed, stop heating, wait for the temperature to drop to room temperature, add 200.0mL dichloromethane solution to the reaction flask, stir well until the solid dissolves, and obtain the dichloromethane solution of intermediate (II);
[0035] S2: Add 42.86 g of p-aminoethylbenzenesulfonamide to the intermediate (II) dichloromethane solution obtain...
Embodiment 2
[0039] S1: Add 50.00g of acrylic acid and 70.21g of thionyl chloride to a 500mL three-necked flask in turn, start stirring, turn on heating, raise the temperature to reflux temperature of 79±1°C, and continue reflux reaction for 1h; after reflux for 1h, cool down slightly until no more reflux , install the distillation device, heat up and evaporate the remaining thionyl chloride, and after 30 minutes, remove the distillation device and change to vacuum distillation to distill the remaining traces of thionyl chloride in the three-necked flask until a small amount of white flocs appear on the condenser. until the solid is formed, stop heating, and when the temperature drops to room temperature, add 200.0mL of dichloromethane solution to the reaction flask, fully stir until the solid dissolves, and obtain the dichloromethane solution of intermediate (II);
[0040] S2: Add 45.27 g of p-aminoethylbenzenesulfonamide to the intermediate (II) dichloromethane solution obtained in step S...
Embodiment 3
[0042]S1: Add 50.00g of acrylic acid and 64.90g of thionyl chloride to a 500mL three-necked flask in turn, start stirring, turn on heating, raise the temperature to reflux temperature of 79±1°C, and continue the reflux reaction for 10h; after reflux for 10h, cool down slightly until no more reflux , install the distillation device, heat up and steam the remaining thionyl chloride, and after 30 minutes, remove the distillation device and use reduced pressure distillation to distill the remaining traces of thionyl chloride in the three-necked bottle until a small amount of white flocs appear on the condenser. until the solid is formed, stop heating, and when the temperature drops to room temperature, add 200.0mL dichloromethane solution in the reaction flask, fully stir until the solid dissolves, and obtain the dichloromethane solution of intermediate (II);
[0043] S2: Add 44.07 g of p-aminoethylbenzenesulfonamide to the intermediate (II) dichloromethane solution obtained in ste...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com