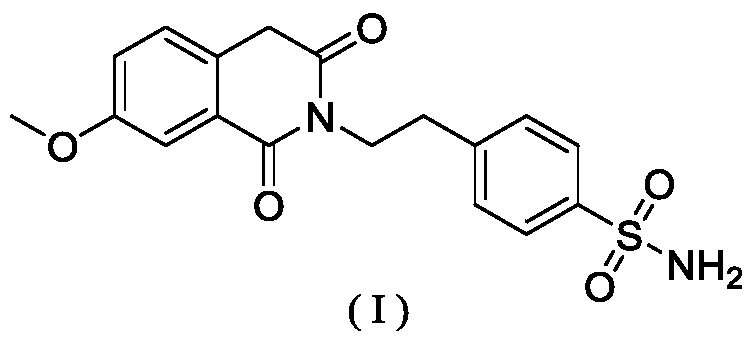
A kind of method for preparing gliquidone intermediate
A technology of gliquidone and intermediates, which is applied in the field of pharmaceutical chemical synthesis, and can solve problems such as safety, hidden dangers in operation, high reaction temperature, and harsh heating conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
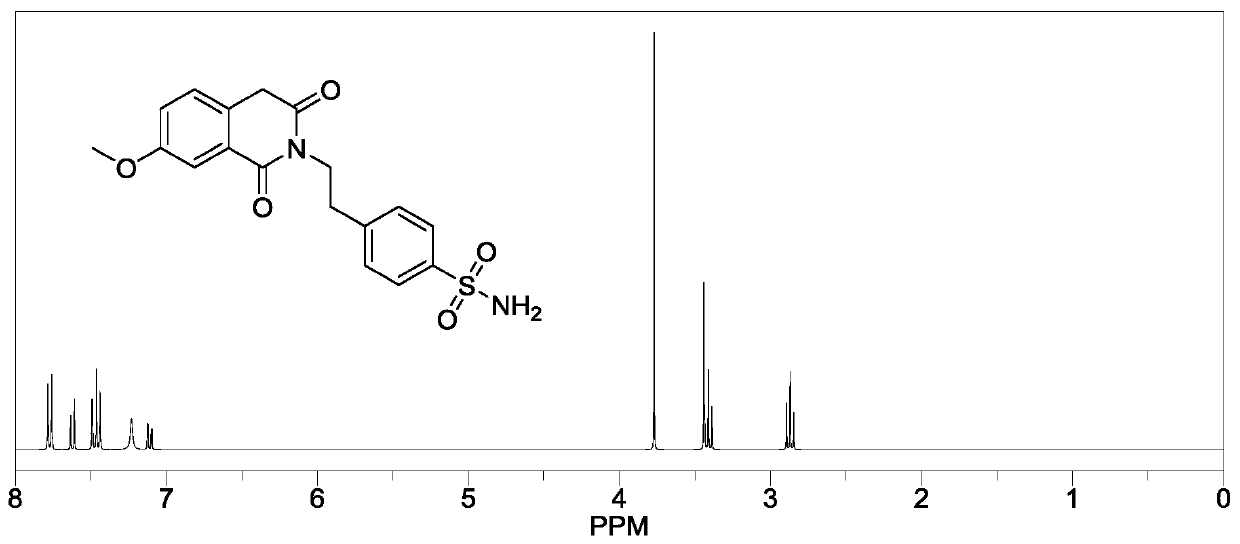
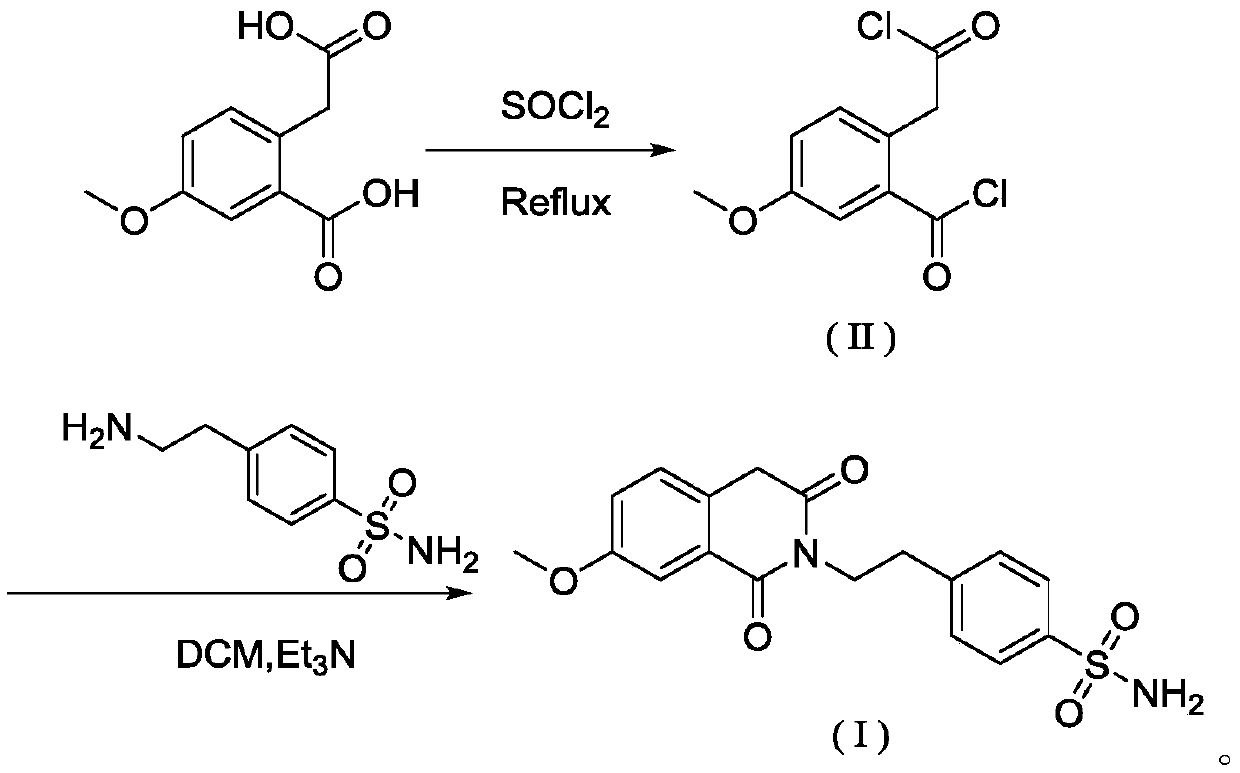
[0034] S1: Add 50.00g of 2-carboxymethyl-5-methoxybenzoic acid and 58.88g of thionyl chloride to a 500mL three-necked flask in turn, start stirring, turn on heating, raise the temperature to reflux temperature of 79±1°C, and continue the reflux reaction for 2h ; After reflux for 2 hours, cool down slightly until no more reflux, install a distillation device, heat up and steam to remove the remaining thionyl chloride, and after 30 minutes, remove the distillation device and use reduced pressure distillation to remove the remaining traces of dichloride in the three-necked flask. sulfoxide, until a small amount of white flocculent solids appear on the condenser, stop heating, wait for the temperature to drop to room temperature, add 200.0mL dichloromethane solution in the reaction flask, fully stir until the solid dissolves, and obtain the dichloromethane of intermediate (II). Chloromethane solution;
[0035] S2: Add 42.86 g of p-aminoethylbenzenesulfonamide to the intermediate (...
Embodiment 2
[0039] S1: Add 50.00g of 2-carboxymethyl-5-methoxybenzoic acid and 70.21g of thionyl chloride to a 500mL three-necked flask in sequence, start stirring, turn on heating, raise the temperature to reflux temperature of 79±1°C, and continue the reflux reaction 1h; after reflux for 1h, lower the temperature slightly until no more reflux, install a distillation device, raise the temperature and heat to evaporate the remaining thionyl chloride, and after 30 minutes, remove the distillation device and use reduced pressure distillation to remove the remaining trace dichloride in the three-necked flask. Thionyl chloride, until a small amount of white flocculent solids appear on the condenser, stop heating, wait for the temperature to drop to room temperature, add 200.0mL dichloromethane solution in the reaction flask, fully stir until the solid dissolves, and obtain the intermediate (II) Dichloromethane solution;
[0040] S2: Add 45.27 g of p-aminoethylbenzenesulfonamide to the interme...
Embodiment 3
[0042]S1: Add 50.00g of 2-carboxymethyl-5-methoxybenzoic acid and 64.90g of thionyl chloride to a 500mL three-necked flask in sequence, start stirring, turn on heating, raise the temperature to reflux temperature of 79±1°C, and continue the reflux reaction 10h; after reflux for 10h, lower the temperature slightly until no more reflux, install a distillation device, heat up and heat to evaporate the remaining thionyl chloride, and after 30 minutes, remove the distillation device, and use reduced pressure distillation to remove the remaining trace dichloride in the three-necked bottle. Thionyl chloride, until a small amount of white flocculent solids appear on the condenser, stop heating, wait for the temperature to drop to room temperature, add 200.0mL dichloromethane solution in the reaction flask, fully stir until the solid dissolves, and obtain the intermediate (II) Dichloromethane solution;
[0043] S2: Add 44.07 g of p-aminoethylbenzenesulfonamide to the intermediate (II) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com