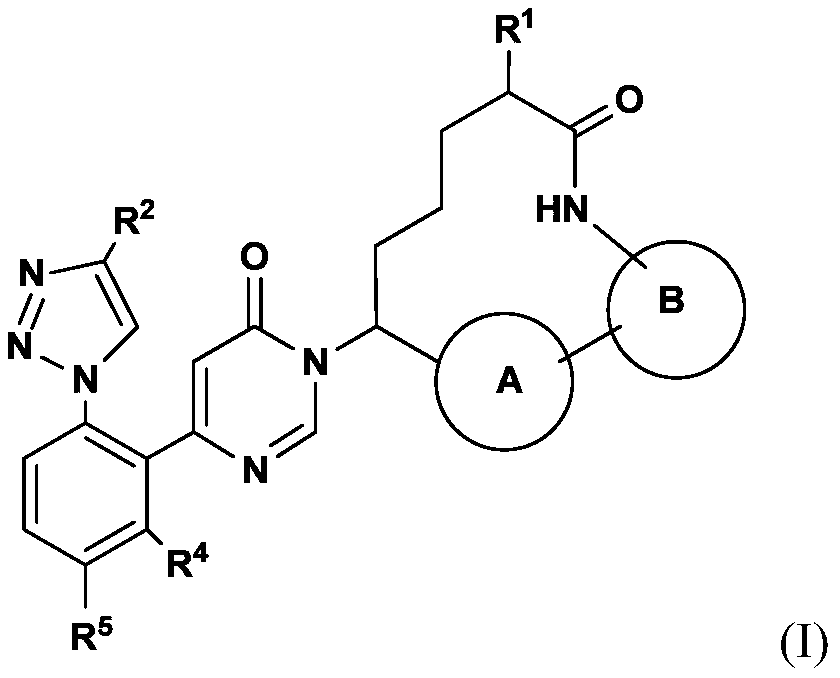
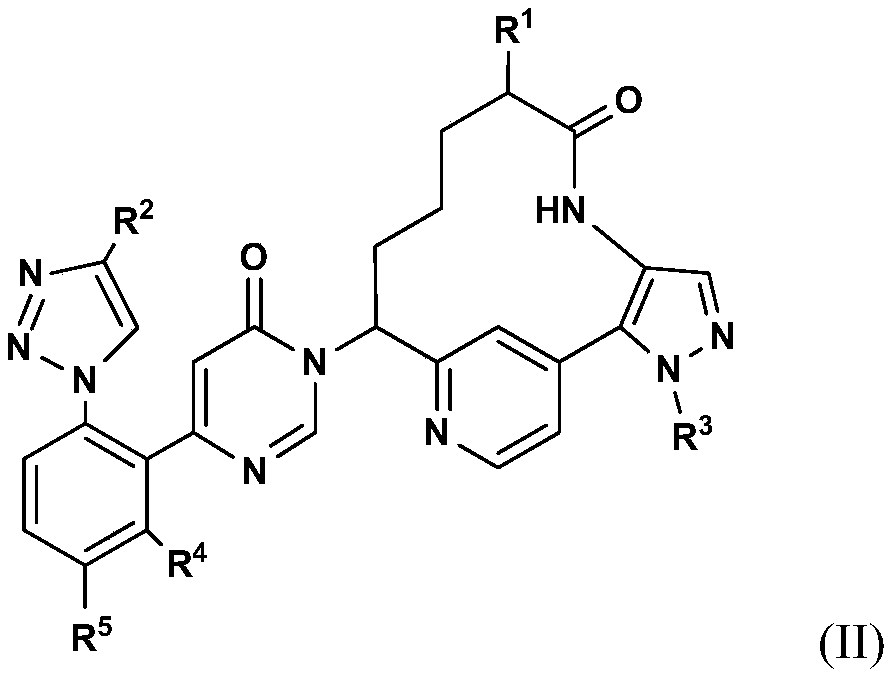
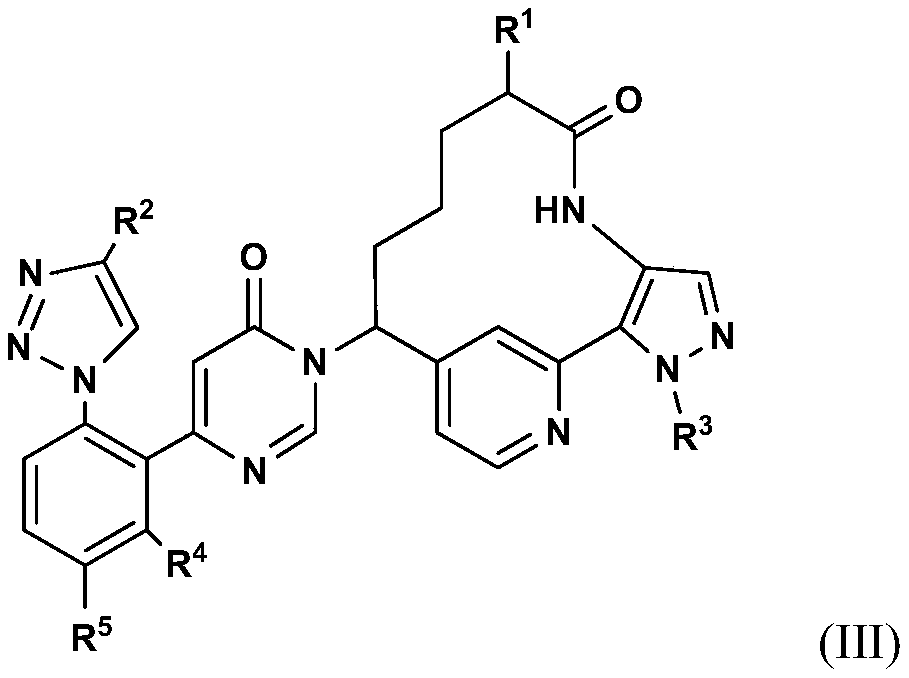
Pyrimidinones as factor xia inhibitors
A technology selected from compounds, applied in medical preparations containing active ingredients, extracellular fluid diseases, drug combinations, etc., can solve problems such as limiting intestinal permeability and limiting oral availability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0087] In another embodiment, the present invention provides a composition comprising at least one compound of the present invention, or a stereoisomer, tautomer, pharmaceutically acceptable salt or solvate thereof.
[0088] In another embodiment, the present invention provides a pharmaceutical composition comprising a pharmaceutically acceptable carrier and at least one compound of the present invention or a stereoisomer, tautomer, pharmaceutically acceptable salt or solvent thereof compounds.
[0089] In another embodiment, the present invention provides a pharmaceutical composition comprising: a pharmaceutically acceptable carrier and a therapeutically effective amount of at least one compound of the present invention or a stereoisomer, tautomer, pharmaceutically acceptable Accepted salts or solvates.
[0090] In another embodiment, the present invention provides methods for preparing compounds of the present invention.
[0091] In another embodiment, the present inventio...
Embodiment 1
[0456] Example 1. (9R,13S)-13-{4-[5-chloro-2-(4-chloro-1H-1,2,3-triazol-1-yl)phenyl]-6-oxo -1,6-dihydropyrimidin-1-yl}-3-(difluoromethyl)-9-methyl-3,4,7,15-tetraazatricyclo[12.3.1.0 2,6 ] Preparation of octadecadecan-1(18),2(6),4,14,16-penten-8-one trifluoroacetate
[0457]
[0458] 1A. Preparation of 1-(difluoromethyl)-4-nitro-1H-pyrazole
[0459] Will Cs 2 CO 3 (14.41 g, 44.2 mmol) was suspended in a solution of 4-nitro-1H-pyrazole (5.00 g, 44.2 mmol) and DMF (40 mL). After heating to 120°C for 5 min, solid sodium 2-chloro-2,2-difluoroacetate (13.48 g, 88 mmol) was added in 10 equal portions over 20 min. After an additional 10 min of heating, the reaction was complete. The mixture was added to a separatory funnel containing 100 mL of water and washed with Et 2 O (2 x 50 mL) was extracted. The combined organic layers were concentrated. Purification by normal phase chromatography eluting with a hexane / EtOAc gradient gave 1-(difluoromethyl)-4-nitro-1H-pyrazole (6.99 ...
Embodiment 2
[0474] Example 2. (9R,13S)-13-{4-[5-chloro-2-(4-chloro-1H-1,2,3-triazol-1-yl)phenyl]-6-oxo -1,6-dihydropyrimidin-1-yl}-9-methyl-4-(pyridin-3-yl)-3,4,7,15-tetraazatricyclo[12.3.1.0 2,6 ] Octadecyl-1(18),2,5,14,16-penten-8-one trifluoroacetate
[0475]
[0476] 2A. Preparation of 4-nitro-1-((2-(trimethylsilyl)ethoxy)methyl)-1H-pyrazole
[0477] To a solution of 4-nitro-1H-pyrazole (5.0 g, 44.2 mmol) in THF (100 mL) was added N-cyclohexyl-N-methylcyclohexylamine (0.948 mL, 4.43 mmol) at 0 °C, Then SEM-Cl (12.55 mL, 70.7 mmol) was added dropwise. The reaction mixture was then gradually warmed to room temperature and stirred overnight at room temperature. The reaction mixture was then concentrated before purification by using normal phase chromatography to give 4-nitro-1-((2-(trimethylsilyl)ethoxy)methyl)-1H-pyrazole (2.4 g, 21% yield). 1 H NMR (500MHz, CDCl 3 )δ8.31(s,1H),8.10(s,1H),5.46(s,2H),3.67-3.55(m,2H),0.99-0.90(m,2H),0.05-0.03(m,9H) .
[0478] 2B. (S)-(1-(4-(4-n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com