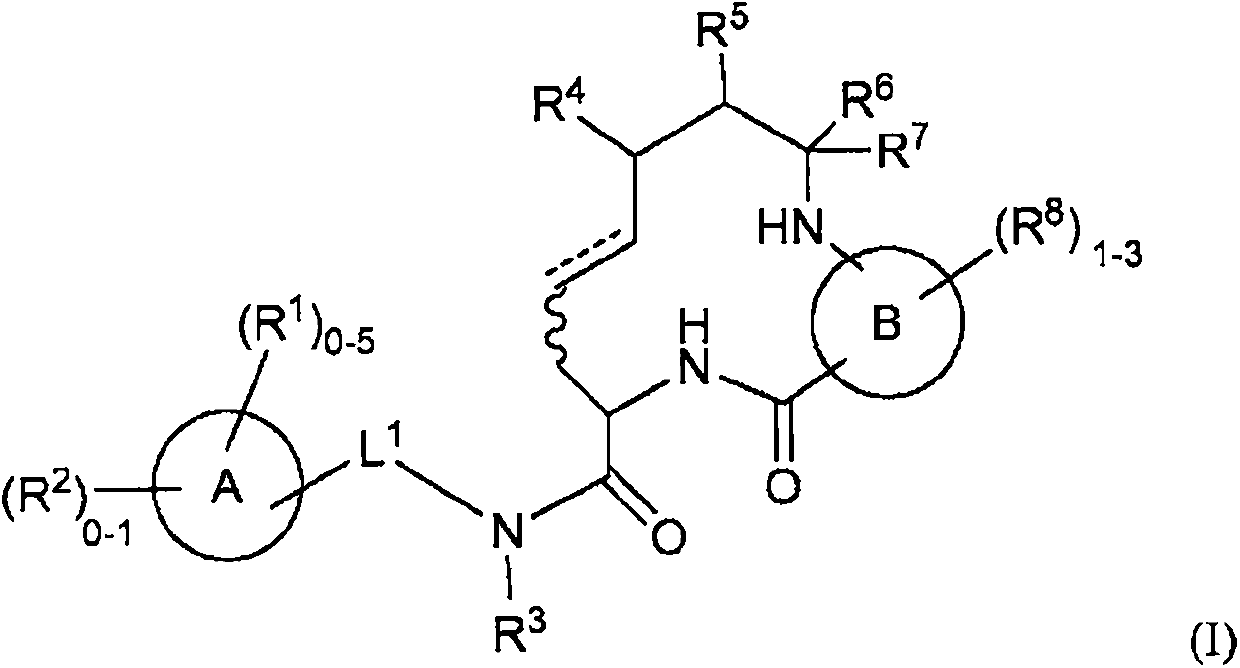
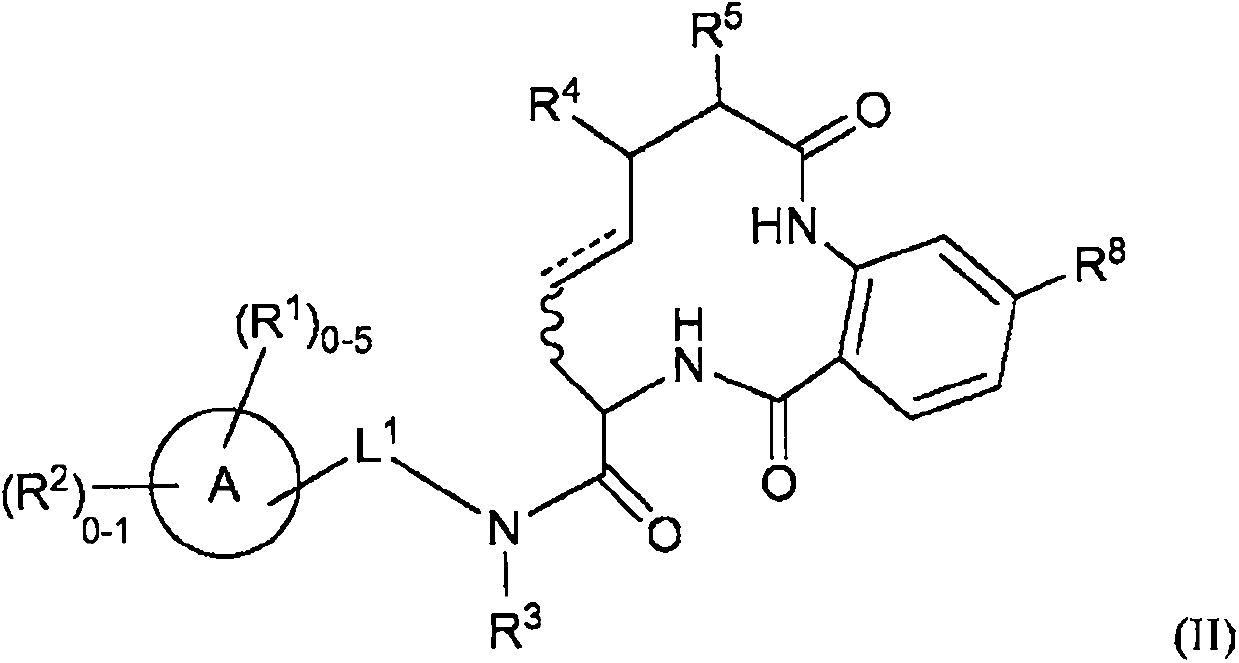
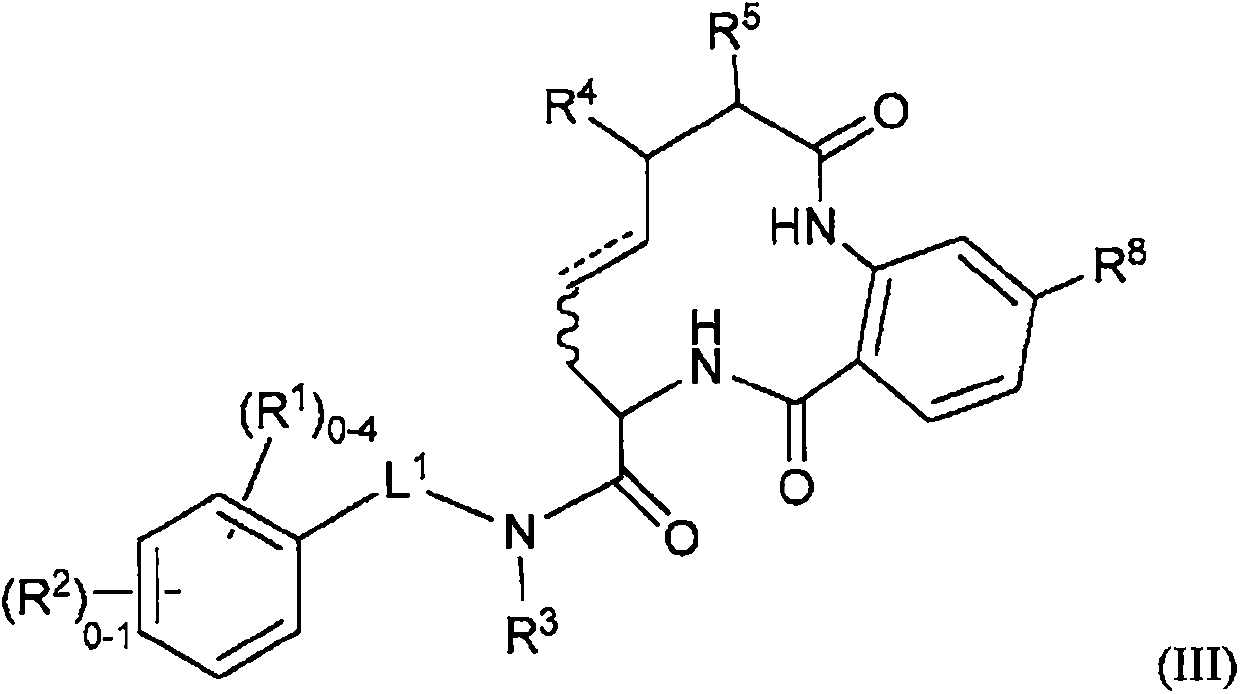
Diamide macrocycles that are FXIA inhibitors
一种化合物、杂环的技术,应用在作为FXIA抑制剂的二胺大环化合物领域,能够解决限制肠渗透、限制可口服性能等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0191] The preparation of prodrugs is known in the art and described in, for example, King, F.D., ed., Medicinal Chemistry: Principles and Practice, The Royal Society of Chemistry, Cambridge, UK (1994); Testa, B. et al., Hydrolysis in Drug and Prodrug Metabolism. Chemistry, Biochemistry and Enzymology, VCHA and Wiley-VCH, Zurich, Switzerland (2003); Wermuth, C.G., Ed., The Practice of Medicinal Chemistry, Academic Press, San Diego, CA (1999) .
[0192] The present invention is intended to include all isotopes of atoms present in the compounds of the present invention. Isotopes include those atoms having the same atomic number but different mass numbers. By way of general example and not limitation, isotopes of hydrogen include deuterium and tritium. Isotopes of carbon include 13 C and 14 c.
[0193] Isotopically labeled compounds of the invention can generally be prepared by conventional techniques known to those skilled in the art or by methods analogous to those describ...
Embodiment 1
[0465] (±)-N-[8-({2-[5-chloro-2-(1H-1,2,3,4-tetrazol-1-yl)phenyl]ethyl}carbamoyl)-2 , 10-dioxo-1,2,3,4,5,6,7,8,9,10-decahydro-1,9-benzodiazepine-13-yl] Methyl carbamate
[0466]
[0467] 1A. (±) 2-Aminopent-4-enoic acid methyl ester HCl salt: To a suspension of 2-aminopent-4-enoic acid (1.9 g, 16.50 mmol) in MeOH (50 mL) at 0 °C Thionyl chloride (2.168 mL, 29.7 mmol) was added dropwise. After the addition, the cooling bath was removed and the reaction was stirred overnight at room temperature. Solvent was removed to afford a white solid.
[0468] 1 H NMR (400MHz, CD 3 OD)δ5.79(dd, J=10.1, 7.5Hz, 1H), 5.42-5.11(m, 2H), 3.84(s, 3H), 2.90-2.35(m, 2H).
[0469] 1B. (±) 2-(2-amino-4-nitrobenzamido)methyl pent-4-enoate: 2-amino-4-nitrobenzoic acid (2.199g, 12.08mmol ) in DMF (24ml) was added 1A (2g, 12.08mmol), HOBT (0.925g, 6.04mmol), DIEA (6.33ml, 36.2mmol) and EDC (2.55g, 13.28mmol). The reaction was stirred at room temperature under Ar for 16 hours. The reaction mixt...
Embodiment 2
[0479] Example 2 (Enantiomer 1) and Example 3 (Enantiomer 2)
[0480] N-[8-({2-[5-chloro-2-(1H-1,2,3,4-tetrazol-1-yl)phenyl]ethyl}carbamoyl)-2,10-di Methyl oxo-1,2,3,4,5,6,7,8,9,10-decahydro-1,9-benzodiazepinedodeca-13-yl]carbamate (enantiomer 1) and
[0481] N-[8-({2-[5-chloro-2-(1H-1,2,3,4-tetrazol-1-yl)phenyl]ethyl}carbamoyl)-2,10-di Methyl oxo-1,2,3,4,5,6,7,8,9,10-decahydro-1,9-benzodiazepinedodeca-13-yl]carbamate (enantiomer 2)
[0482]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com