Human endothelin type a receptor immunogenic peptide and its carrier vaccine
An immunogenic, carrier-based vaccine technology, applied in the direction of hormone receptors, receptors/cell surface antigens/cell surface determinants, carrier-bound antigens/hapten components, etc., can solve problems such as non-identity, and achieve the goal of enhancing immune response Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: ET A Preparation of R immunogenic peptides
[0024] According to the characteristics of bioinformatics technology, such as amino acid hydrophilicity, spatial conformation, and B cell epitope, the ET A ET of R extracellular amino acid sequence A 9 R immunogenic peptides, respectively named HI10, RF9, EC10, YC9, RC8, EM7, TF10, CK8, NE9, the specific amino acid sequences are, HI10: SEQ ID No.1; RF9: SEQ ID No.2 ; EC10: SEQ ID No.3; YC9: SEQ ID No.4; RC8: SEQ ID No.5; EM7: SEQ ID No.6; TF10: SEQ ID No.7; CK8: SEQ ID No.8, NE9: SEQ ID No. ID No. 9.
[0025] The above-mentioned 9 peptides were synthesized by PSSM-8 automatic peptide synthesizer (SHIMADZU company, Japan), and the purity of the 9 peptides was analyzed by high performance liquid chromatography, and the purity of the 9 peptides was tested. Reach more than 92%. The obtained 9 peptides were freeze-dried, aliquoted, placed in cryopreservation tubes, and stored at -80°C for later use.
Embodiment 2
[0026] Example 2: Preparation of ET A R immunogenic vector vaccine
[0027] 1. Using Qβ-2aa bacteriophage virus-like particle protein to prepare 9 kinds of vector vaccine ET A R RF9-Qβ, ET A REC10-Qβ, ET A R YC9-Qβ, ET A R RC8-Qβ, ET A R EM7-Qβ, ET A R TF10-Qβ, ET A R CK8-Qβ, ET A R HI10-Qβ, ET A RNE9-Qβ. Concrete preparation process is as follows:
[0028] 1) Preparation of Qβ-2aa bacteriophage VLP: the English abbreviation of Qβ-2aa phage VLP is: Qβ-2aa VLP, hereinafter Qβ-2aa VLP is used to represent Qβ-2aa phage VLP.
[0029] The preparation method of Qβ-2aa VLP is:
[0030] 1a) Obtaining a recombinant strain expressing Qβ-2aa VLP: the recombinant strain is Escherichia coli DH5α / pGEXQβ-A1, and this recombinant strain can induce the production of Qβ-2aa VLP protein. The preservation number of Escherichia coli DH5α / pGEXQβ-A1 is CCTCCNO: M209282. For the specific preparation process, please refer to the Chinese patent: A Qβ-2aa bacteriophage virus-like particle pr...
Embodiment 3
[0044] Example 3ET A In Vitro Functional Study of Antibodies Targeted by R Immunogenic Vector Vaccines
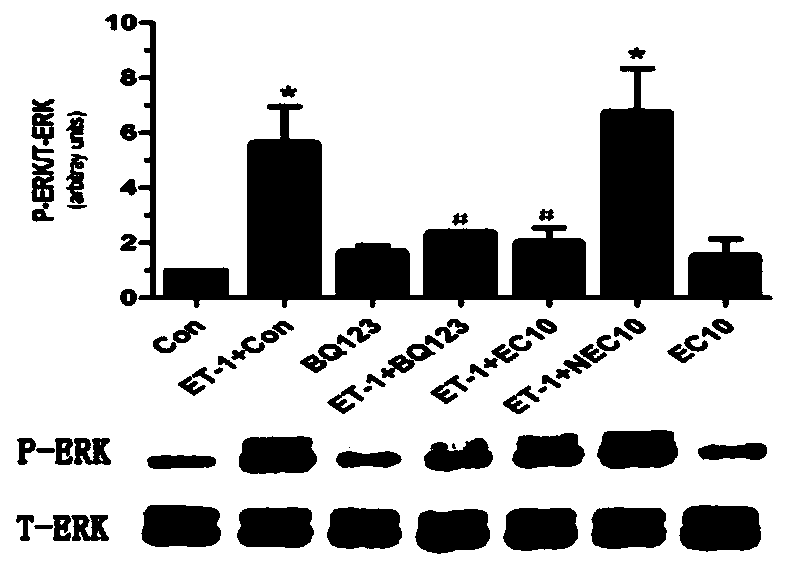
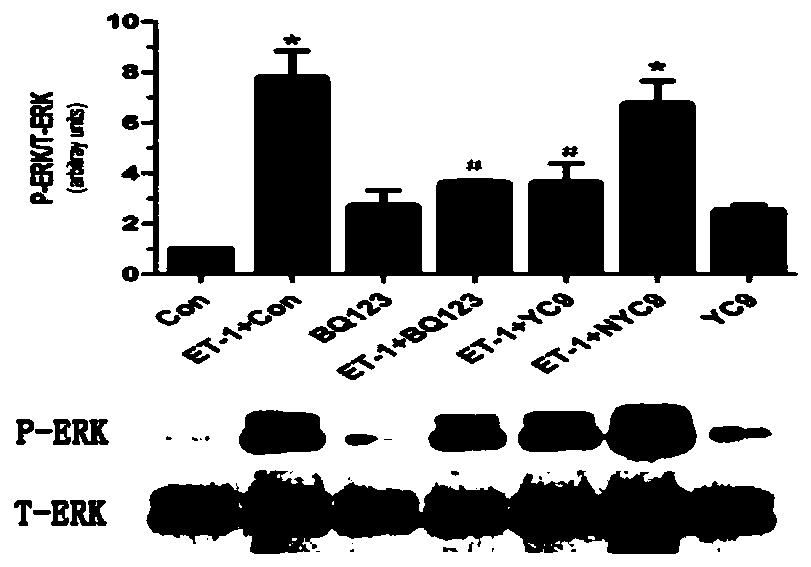
[0045] 1. ET A Screening of R immunogenic peptides: ET is preferred A R immunogenic peptides are EC10, YC9, RC8, EM7, TF10. The specific test process is:
[0046] 9 kinds of ET that embodiment 2 obtains A R immunogenic vector vaccine: ET A R RF9-Qβ, ET A R EC10-Qβ, ET A RYC9-Qβ, ET A R RC8-Qβ, ET A R EM7-Qβ, ET A R TF10-Qβ, ET A R CK8-Qβ, ET A R HI10-Qβ, ET A R NE9-Qβ subcutaneously immunized male New Zealand white rabbits at multiple points on days 1, 14, and 28. Among them, as a control group, 2 male New Zealand white rabbits were immunized with vector Qβ-2aa-CPG-ODN VLP, and each ET A R immunogenic carrier vaccine immunized 2 male New Zealand white rabbits, a total of 20 immunized male New Zealand white rabbits, and the immunization dose was 500ug per rabbit. On the 7th, 14th, 21st, 28th, and 35th days, the blood was taken to measure the antibody titer by E...
PUM
| Property | Measurement | Unit |
|---|---|---|
| antibody titer | aaaaa | aaaaa |
| antibody titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com