SARS nucleic acid vaccine and preparation method, application of S gene in coronavirus
A nucleic acid vaccine and vaccine technology, applied in the fields of botanical equipment and methods, biochemical equipment and methods, pharmaceutical formulations, etc., can solve problems such as infection and lack of vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation of SARS nucleic acid vaccine:
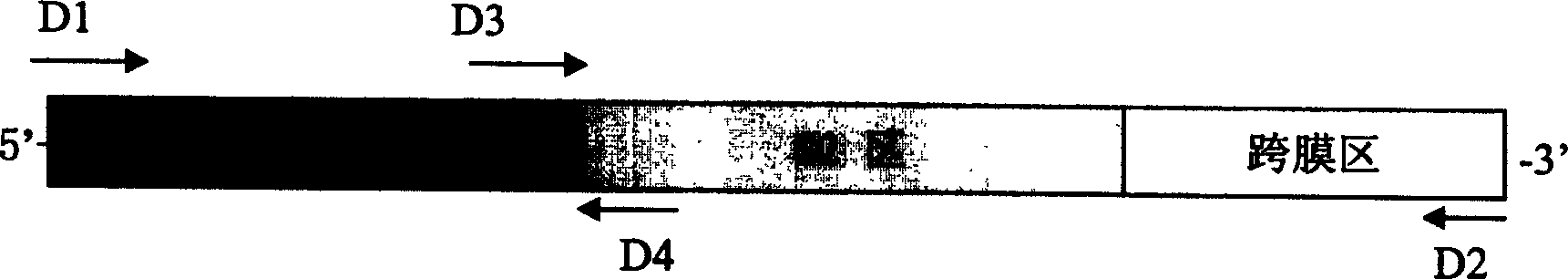
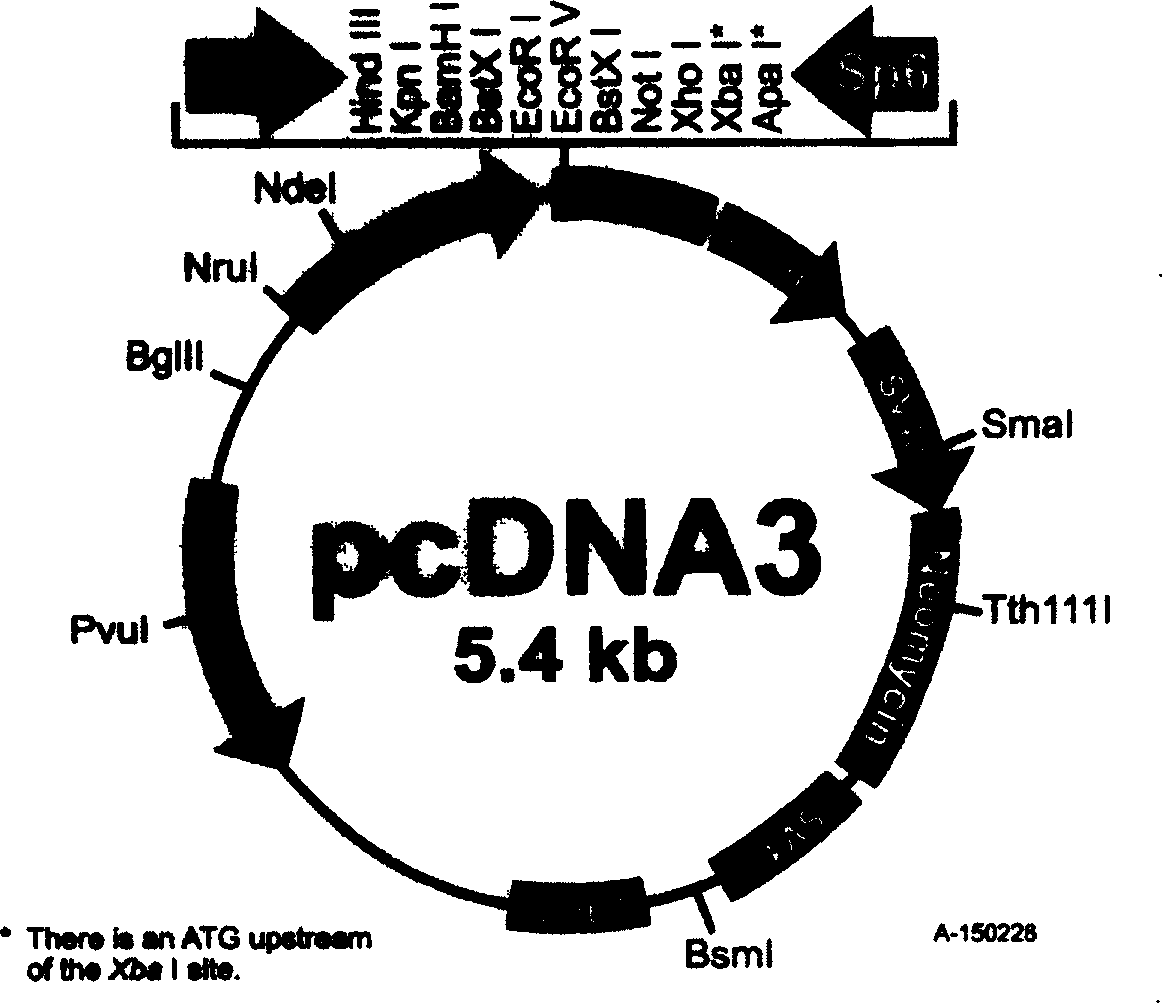
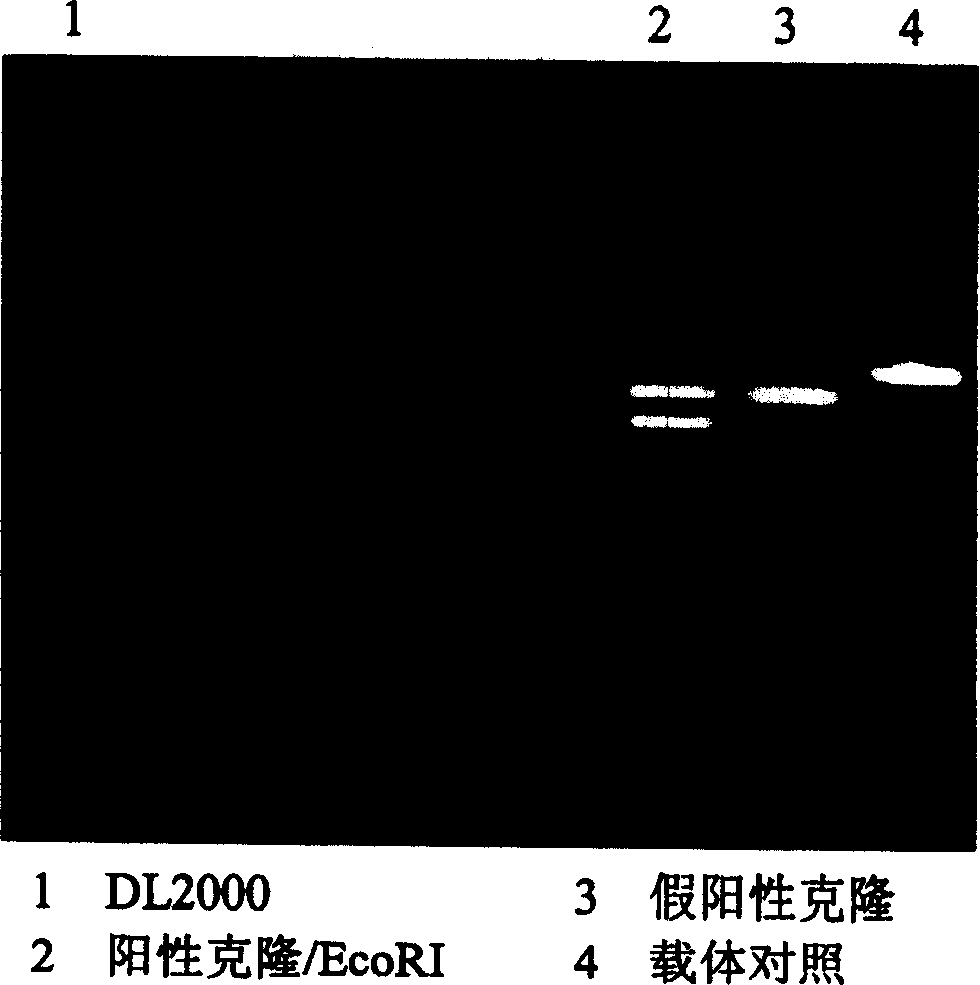
[0036] Get the SARS-related coronavirus sPike(S F , S N , S M , S C ) gene, amplified by PCR method, after PCR, digested with EcoR1, and simultaneously digested pcDNA3 (see image 3 , Figure 4 ), connected, transformed into Escherichia coli, using ampicillin (Amp r ) resistance screening positive clones (see Figure 5 , Image 6 ), cultivated, purified, and prepared to obtain the SARS nucleic acid vaccine.
[0037] The dosage form is injection.
[0038] The SARS vaccine contains SARS-associated coronavirus S gene and pcDNA3 plasmid. Cloned to pcDNA3 is the full length of the S gene of SARS-associated coronavirus.
[0039] Cloned into the eukaryotic expression plasmid is SARS-associated coronavirus S gene S 1 region segment.
[0040] Cloned into the eukaryotic expression plasmid is SARS-associated coronavirus S gene S 2 region segment.
[0041] Cloned into the eukaryotic expression plasmid is SARS-associated coro...
Embodiment 2
[0044] The N-terminal fragment of the S gene of SARS-associated coronavirus was cloned into pcDNA3. All the other are with embodiment 1.
Embodiment 3
[0046] The middle fragment of the S gene of SARS-associated coronavirus was cloned into pcDNA3. All the other are with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com