Genetic transformation method of larch embryonic cells
A genetic transformation method, embryogenic cell technology, applied in sterilization methods, biochemical equipment and methods, methods of supporting/immobilizing microorganisms, etc., can solve the problems of lack of system, slow progress of genetic transformation research, lack of regeneration system, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Example 1, Construction of recombinant plasmid pSuper::MIR156 and preparation of recombinant Agrobacterium GV3101 / MIR156
[0081] 1. Construction of recombinant plasmid pSuper::MIR156
[0082] 1. Design primer pairs
[0083] The coding sequence of the miR156 precursor is shown in Sequence 1 of the Sequence Listing, and a primer pair (composed of M156F and M156R) was designed at a position greater than 90 bp at both ends of the stem-loop structure sequence.
[0084] M156F: 5'-CCC AAGCTT TTGTACTCAGCCGACAGAA-3';
[0085] M156R: 5'-GG ACTAGT CCTCTAGCGGTAAATCTCAA-3'.
[0086] 2. Genomic DNA of Japanese larch was extracted as a template, and the composition of M156F and M156R was used for PCR amplification to obtain PCR amplification products.
[0087] 3. Digest the PCR amplification product obtained in step 2 with restriction endonucleases Hind III and Spe I, and recover the digested product.
[0088] 4. Digest the Super1300 vector with restriction endonucleases Hind...
Embodiment 2
[0092] Embodiment 2, genetic transformation of larch
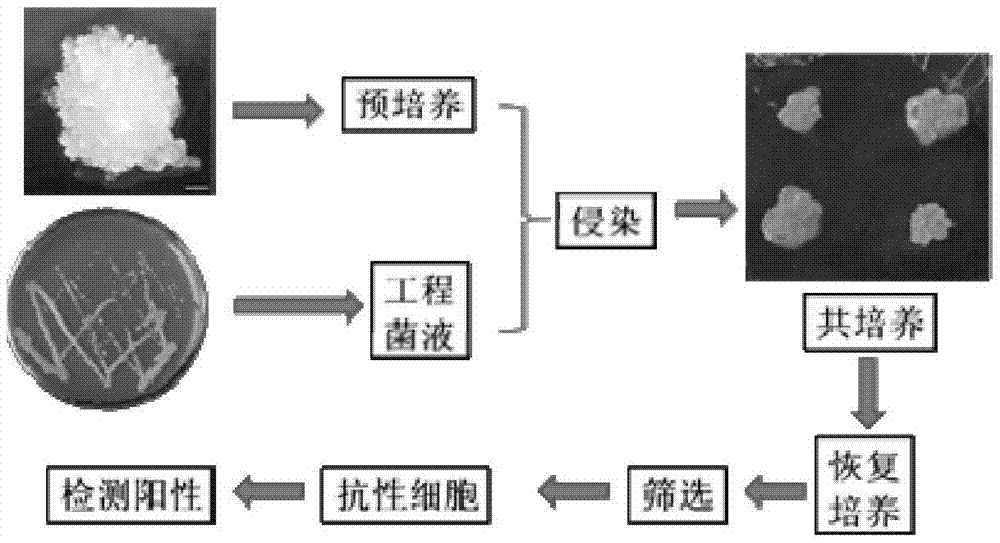
[0093] For the flow chart of genetic transformation, see figure 1 .
[0094] 1. Preparation and pre-cultivation of receptor materials
[0095] 1. Inoculate Japanese larch embryogenic cells into S 0 On the culture medium, cultured statically at 23°C and in the dark for 15 days.
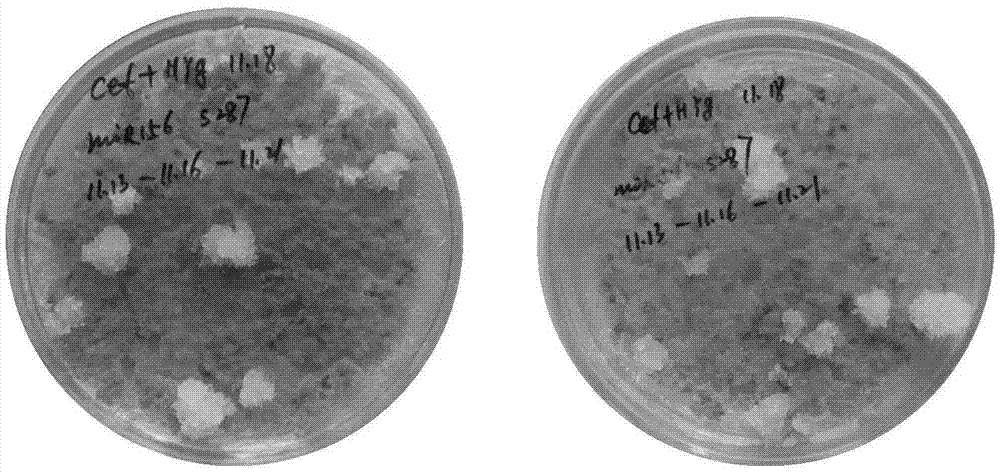
[0096] 2. After completing step 1, pick about 1g of embryogenic cells with vigorous growth on the surface, and add 92ml Y 0 In a 250ml Erlenmeyer flask with culture medium, shake culture at 100rpm under 23°C and dark conditions. Subculture once every 7 days (the method of each subculture is as follows: get about 1g of embryogenic cells, add 92ml Y 0 medium in a 250ml Erlenmeyer flask, 23°C, 100rpm shaking culture under dark conditions). On the 7th day of subculture, 1 mg of acetosyringone was added to each flask, and the timing was started from the addition of acetosyringone. After 12 hours, embryogenic cells were taken as the recipient mater...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com