Application of a rifamycin-quinazinone dual target molecule
A technology of rifamycin and quinazinone is applied in the field of medicinal chemistry and achieves the effect of good application prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
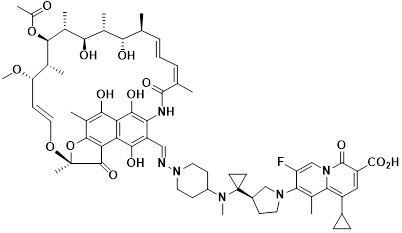
[0031] This example provides the application of the rifamycin-quinazinone dual target molecule represented by formula I in inhibiting anaerobic bacteria;
[0032]
[0033]Among them, anaerobic bacteria include Actinomyces naeseri, Anaerobic coccus praustzii, Atobo vaginalis, Bacteroides fragilis (including metronidazole-resistant type), Bacteroides polymorpha (including metronidazole-resistant type ), Bacteroides cellulosus, Bacteroides monomorpha, Bacteroides vulgaris, Bacteroides ovale (including metronidazole-resistant types), Bifidobacterium breve, Bifidobacterium longum, Clostridium sporeformans, Perfringens Clostridium difficile (including metronidazole-resistant type), Eubacterium rectum, Fusobacterium nucleatum, Gardnerella vaginalis, Lactobacillus crispatus, Lactobacillus gasseri, Lactobacillus jansnii, Mobilis kervii, Campylobacter shy, Peptococcus (Niger), Glycopeptophilus (including metronidazole-resistant), Peptostreptococcus large, Peptostreptococcus anaerobic...
Embodiment 2
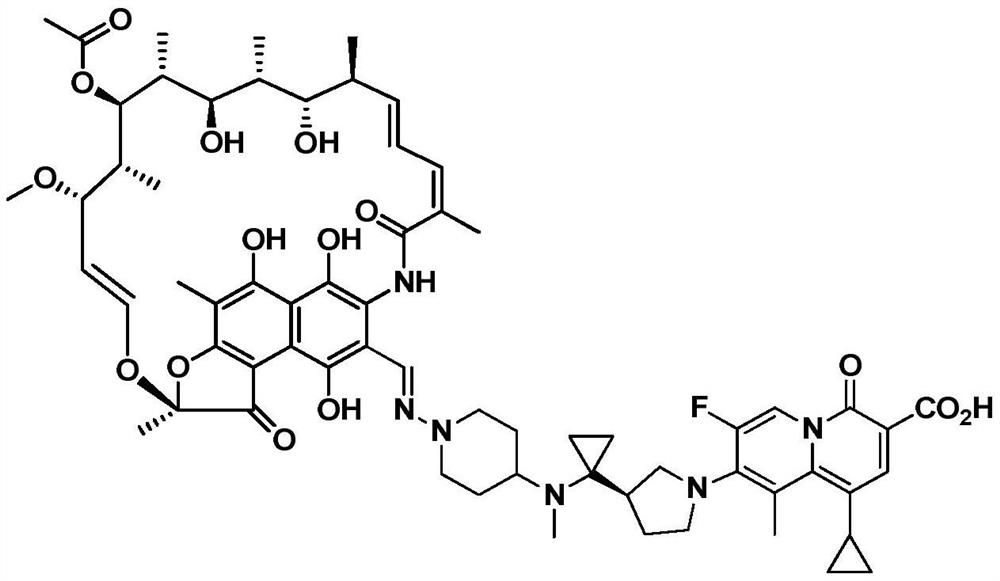
[0053] This example provides the prescription and preparation method of a fast-release oral preparation of the rifamycin-quinazinone dual target molecule represented by formula I.
[0054]
[0055] Weigh the rifamycin-quinazinone dual-target molecule and excipients represented by formula I according to the above prescription. Dissolve povidone K30 (PVP K30) and sodium dodecyl sulfate (SDS) in purified water, stir for 1 hour, and use it as an adhesive for later use; Pass through a 30-mesh sieve, add to the granulator, and pre-mix, the impeller stirring speed is 700rpm, and the time is about 15 minutes. Then use a peristaltic pump to add an appropriate amount of purified water and binder to the mixture of the granulator at a fixed speed (145-165g / min). The stirring speed of the impeller of the granulator is 400rpm, and the time is about 1 to 2 minutes. After the binder is added , continue mixing for 0.5 to 1 minute; use a fluidized bed to dry the wet granules, set the inlet ...
Embodiment 3
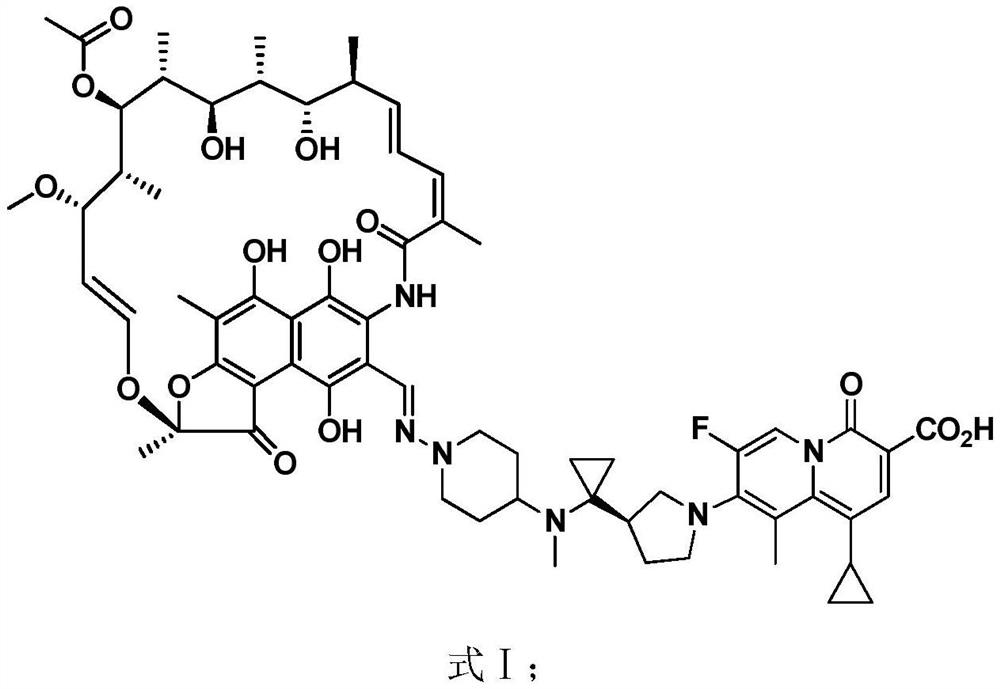
[0058] This example provides a method for preparing an injection of the rifamycin-quinazinone dual target molecule represented by formula I.
[0059]
[0060] Add mannitol, acetaldehyde sodium sulfoxylate, and Tween-80 to an appropriate amount of water for injection under nitrogen protection, add the rifamycin-quinazinone dual-target molecule shown in formula I, and stir at a medium speed for 10-15 minutes. Wet the rifamycin-quinazinone dual-target molecule shown in formula I, and slowly add 1N NaOH dropwise, which takes about 175 minutes (fast at the beginning and slow at the end), to the rifamycin-quinazinone shown in formula I The dual-target molecules are completely dissolved, filtered through two microporous membranes of 0.45+0.22μm, and the filtrate is filled into 10mL glass bottles, each containing 3.5mL, and the glass bottles are transferred to a lyophilizer for lyophilization, and the formula is obtained after capping The freeze-dried powder injection of the rifamy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com