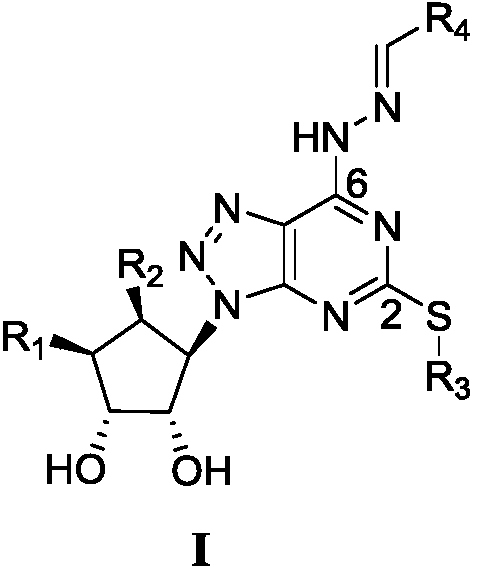
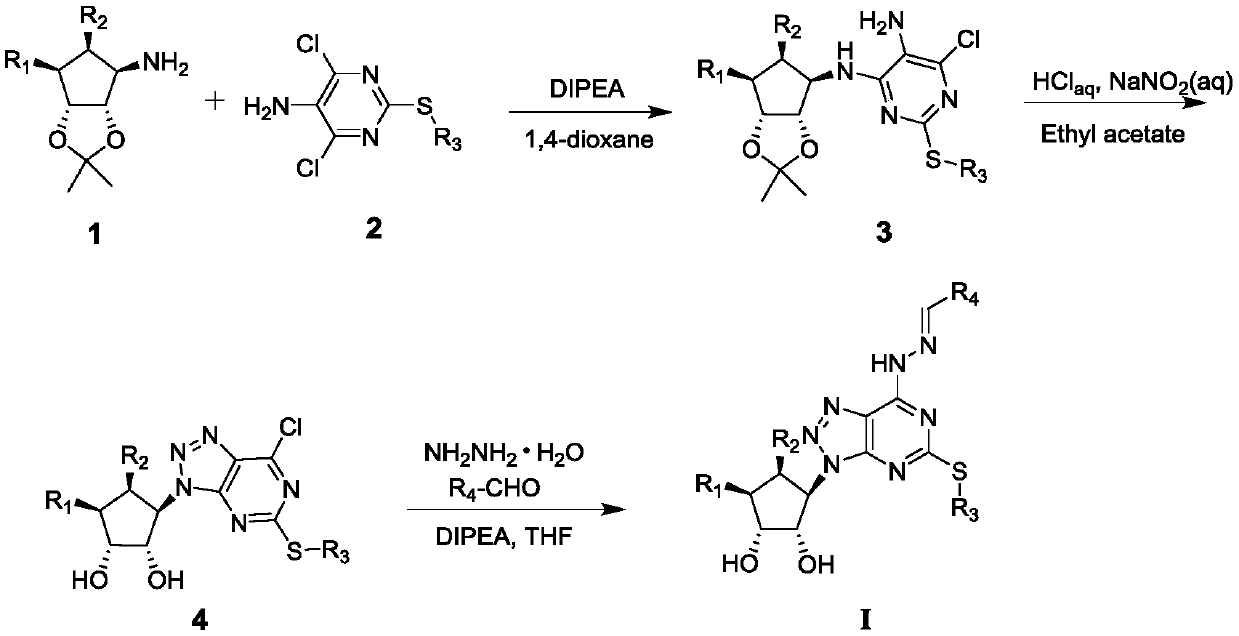
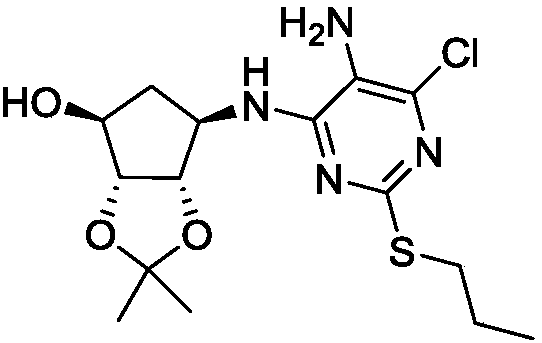
6-hydrazone-8-azapurine compound and its preparation method and application
A technology of heteropurines and compounds, applied in the field of 6-hydrazone-8-azapurine compounds and their preparation, can solve problems such as dyspnea and limit the wide-scale use of ticagrelor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (1)(1S,2R,3S,4R)-6-[[5-amino-6-chloro-2-(propylthiouracil)-4-yl]-amino]-2,2-dimethyl Preparation of tetrahydro-4H-cyclopentyl[d][1,3]dioxan-4-ol
[0023] Add 4,6-dichloro-5-amino-2-propylthiopyrimidine (16.10g, 0.068mol) to 300ml 1,4-dioxane, add 2-[[(3R,4S ,6R,6S)-6-aminotetrahydro-2,2-dimethyl-4H-cyclopenta-1,3-dioxol-4-yl]oxy]-alcohol mandelate (22.03g, 0.075mol) and N,N-diisopropylethylamine (43.94g, 0.34mol), heated to 100°C for reaction, the color of the reaction system gradually changed from yellow to brown, and the reaction progress was monitored by thin-layer chromatography. After 26 hours, After the basic reaction of the system is completed, cool down to room temperature, then add 500ml of water, extract, separate the liquids, extract the water phase twice with ethyl acetate, combine the organic phases, wash once with saturated saline, and dry the organic phases with anhydrous magnesium sulfate. Then the organic solvent was distilled off under reduced pressu...
Embodiment 2
[0035] 9-[(1'R,2'S,3'R,4'S)-2',3',4'-trihydroxycyclopent-1'-yl]-9H-2-propylthio-6-(3, 4-difluorobenzaldehyde hydrazone)-8-azapurine (Ib)
[0036]
[0037] The preparation method is similar to the step (3) of Example 1. White solid, melting point 153.6-155.0°C. 1 H NMR (400MHz, DMSO-d 6 ):δ(ppm)1.02(3H,t,-CH 3 ,J=7.2Hz),1.73(2H,m,CH 2 ),1.98(1H,m,5'-CH 2 ),2.63(1H,m,5'-CH 2 ),3.15(2H,CH 2 ),3.82(1H,s,CH),3.97(1H,s,CH),4.72(1H,m,CH),4.93(1H,OH),5.03(1H,m,CH),5.11(1H,OH ), 5.14 (1H, OH), 7.57 (1H, m, Ph-H), 7.71 (1H, s, Ph-H), 7.94 (1H, s, Ph-H), 8.23, 8.49 (1H, =CH ),11.06,11.49(1H,NH).HRMS(ESI)for[M+H] + :calcd 466.14674,found466.14656.
Embodiment 3
[0039] 9-[(1'R,2'S,3'R,4'S)-2',3',4'-trihydroxycyclopent-1'-yl]-9H-2-propylthio-6-(2- Hydroxybenzaldehyde (hydrazone)-8-azapurine (Ic)
[0040]
[0041] The preparation method is similar to the step (3) of Example 1. Yellow solid, melting point 231.9-232.8°C. 1 H NMR (400MHz, DMSO-d 6 ): δ (ppm) 1.01 (3H, t, J = 7.3Hz), 1.72 (2H, m), 1.98 (1H, ddd, J = 13.5, 9.0, 4.4Hz), 2.64 (1H, ddd, J = 13.5 ,9.0,4.4Hz),3.15(2H,m),3.81(1H,dd,J=4.7,4.0Hz),3.96(1H,ddd,J=7.0,7.0,4.4Hz),4.73(1H,ddd, J=9.0,4.7,4.0Hz), 4.99(1H,d,J=4.0Hz),5.04(1H,q,J=9.0Hz),5.11(1H,d,J=7.0Hz),5.14(1H, d, J = 4.0Hz), 6.94 (2H, m), 7.32 (1H, t, J = 7.7Hz), 7.46 (1H, d, J = 7.6Hz), 8.42, 8.69 (1H, = CH), 11.06 ,11.49(1H,NH),12.67(1H,OH). 13 C NMR (100MHz, DMSO-d 6 ): δ (ppm) 13.76, 22.83, 32.74, 36.38, 61.68, 73.65, 74.97, 77.27, 117.41, 118.60, 119.83, 122.87, 131.41, 131.87, 148.74, 151.44, 152.81, 169.37 M+H] + :calcd446.16050,found446.16021.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com