Identification of novel anti-persister activity for borrelia burgdorferi
A technology of Borrelia and spirochetes, which is applied in the field of identification of novel antiviral activity of Borrelia burgdorferi, and can solve the problems of inability to be screened by high-throughput, lengthy measurement, high background and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] Method for identifying novel antidepressant activity of Borrelia burgdorferi
[0127] Bacterial strains, media and cultures: Borrelia burgdorferi strain B31 was obtained from the American Type Tissue Collection. Borrelia burgdorferi was cultured in BSK-H medium (HiMedia Laboratories Pvt. Ltd.) containing 6% rabbit serum (Sigma-Aldrich, St. Louis, MO). All media were filter sterilized with a 0.2-μm filter. Cultures were incubated at 33°C in sterile 50-mL sealed conical tubes (BD Biosciences, San Diego, CA) without antibiotics. After 7 days, 100 μL of stationary phase B. burgdorferi culture (1×10 6 cells) was transferred to 96-well tissue culture microplates for drug selection.
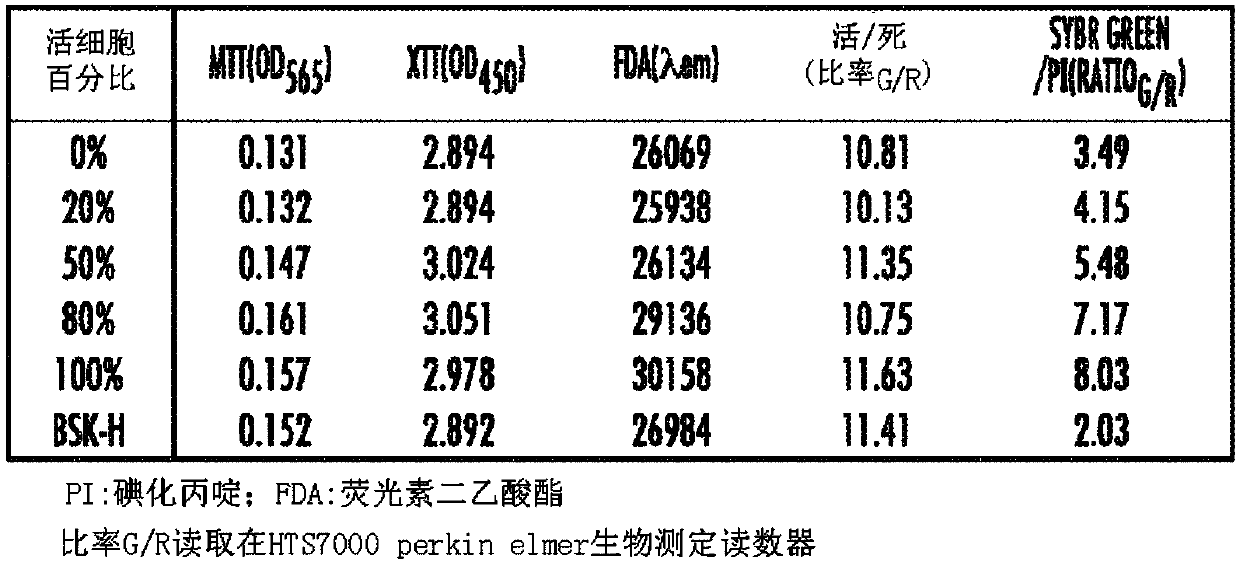
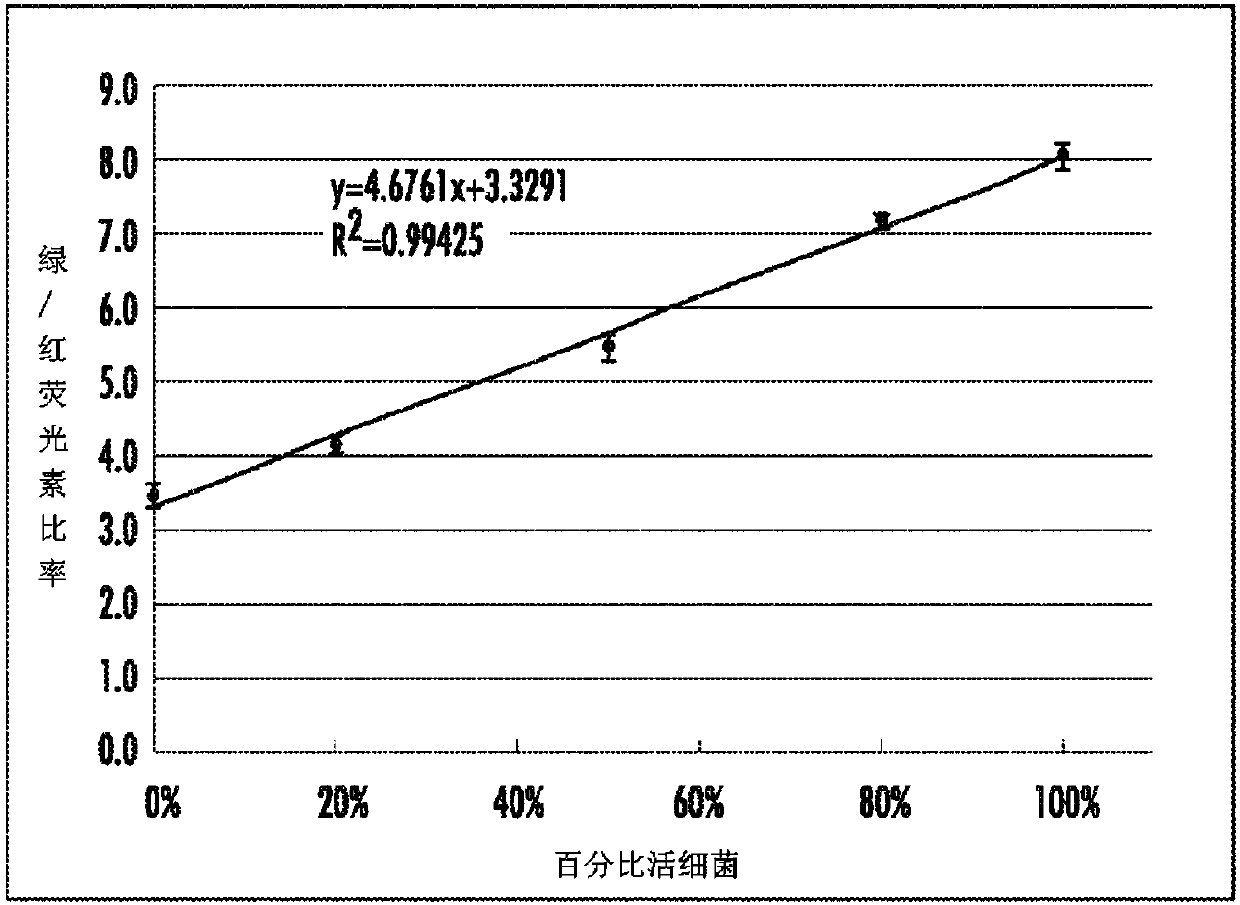
[0128] Microscopy: Samples were examined on a Nikon Eclipse E800 microscope equipped with differential interference contrast (DIC) and epifluorescence illumination, and recorded with a Spot slider color camera. Cell proliferation assays were performed by direct counting using a bacterial cou...
Embodiment 2
[0164] Drug combination on Borrelia survival in vitro: eradication
[0165] Achieved with daptomycin, cefoperazone, and doxycycline
[0166] Materials and methods
[0167] Strains, media and cultures: Strains, media and cultures were obtained and used according to Example 1.
[0168] Antibiotics: Doxycycline (Dox), Amoxicillin (Amo), Cefoperazone (CefP), Clofazimine (Cfz), Miconazole (Mcz), Polymyxin B (Pmb), Sulfamethazine Oxazole (Smx), daptomycin (Dap), carbomycin (magnamycinA), nisin, carbenicillin, ofloxacin, tigecycline, hydroxychloroquine, rifampicin, and clarithromycin (Sigma-Aldrich) dissolved in a suitable solvent (Clinical and Laboratory Standards Institute, 2007). Antibiotic stocks were filter sterilized through a 0.2 μm filter, except for clofazimine, which was dissolved in DSMO (dimethyl sulfoxide) and not filtered. The stock was then stored at -20°C.
[0169] Microscopy: Samples were examined on a Nikon Eclipse E800 microscope equipped with differenti...
Embodiment 3
[0199] FDA-approved drug in circular form effective against Borrelia burgdorferi
[0200] Materials and methods
[0201] Strains, media and cultures: Borrelia burgdorferi strain B31 obtained from the American Type Culture Collection. Borrelia burgdorferi was cultured in BSK-H medium (HiMedia Laboratories Pvt. Ltd) with 6% rabbit serum (Sigma-Aldrich, Co). All media were filter sterilized through 0.2 μM filters. Cultures were incubated at 33°C without antibiotics in sterile 50 mL closed conical tubes (BD Biosciences, California, USA).
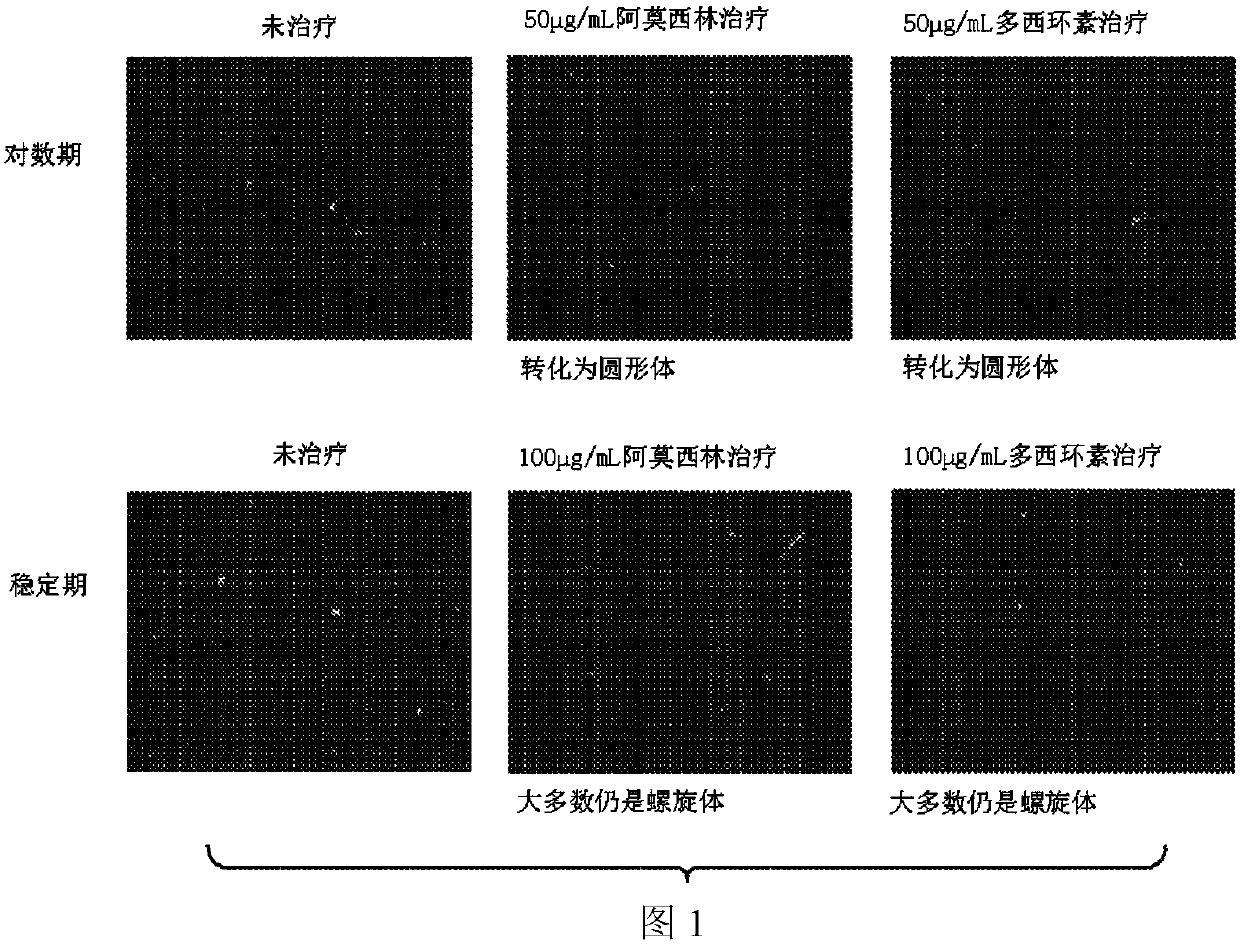
[0202] Induction of the Circular Body Form of Borrelia burgdorferi: To induce the Circular Body Form of Borrelia burgdorferi, Borrelia burgdorferi (1 x 10 5 spirochetes / ml) were cultured in BKS-H medium for 6 days without shaking. After 6 days of incubation, amoxicillin at a final concentration of 50 [mu]g / mL was added to the cultures for induction of the round body form. After induction at 33°C for 72 hours, the rounded form of Borrelia bu...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap