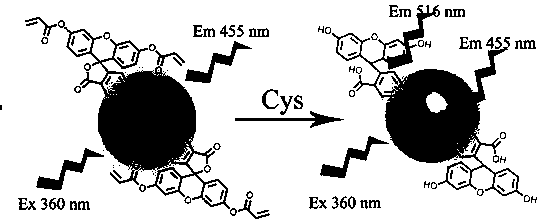
Preparation and application of a water-soluble fluorescent sensor capable of ratiometric detection of cysteine
A fluorescent sensor, cysteine technology, used in fluorescence/phosphorescence, instruments, luminescent materials, etc., can solve problems such as water solubility, limited application, biological toxicity, etc., achieve high selectivity, obvious selectivity, and convenient post-processing Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
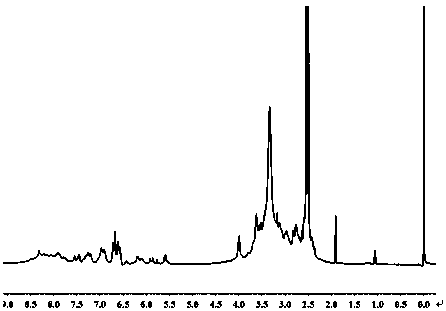
[0025] Example 1: The preparation of a fluorescent sensor capable of ratiometric detection of cysteine, the specific steps are as follows:
[0026] (1) Dissolve fluorescein isothiocyanate (100 mg) and triethylamine (100 mg) in 40 mL of dichloromethane, then dissolve acryloyl chloride (231 mg) in 5 mL of dichloromethane, then Add it dropwise to the above solution, stir at room temperature for 3 h, remove most (85-95%) of dichloromethane by rotary evaporation after the reaction is completed, purify the product through a column, and dry it in vacuum to obtain product 1;
[0027] (2) Dissolve the product 1 (50 mg) synthesized in step (1) and carbon quantum dots (50 mg) prepared according to the prior art in a solution of dimethyl sulfoxide (5 mL), react at room temperature for 24 hours, and react After the end, it was first precipitated with ether, then with methanol, centrifuged, and vacuum-dried to obtain product 2.
Embodiment 2
[0028] Example 2: The preparation of a fluorescent sensor capable of ratiometric detection of cysteine, the specific steps are as follows:
[0029](1) Dissolve fluorescein isothiocyanate (100 mg) and triethylamine (100 mg) in 40 mL of dichloromethane, then dissolve acryloyl chloride (231 mg) in 5 mL of dichloromethane, then Add it dropwise to the above solution, stir at room temperature for 3 h, remove most (85-95%) of dichloromethane by rotary evaporation after the reaction is completed, purify the product through a column, and dry it in vacuum to obtain product 1;
[0030] (2) Dissolve the product 1 (250 mg) synthesized in step (1) and carbon quantum dots (50 mg) prepared according to the prior art in dimethyl sulfoxide (8 mL) solution, react at room temperature for 24 hours, and react After the end, it was first precipitated with ether, then with methanol, centrifuged, and vacuum-dried to obtain product 2.
Embodiment 3
[0031] Example 3: The preparation of a fluorescent sensor capable of ratiometric detection of cysteine, the specific steps are as follows:
[0032] (1) Dissolve fluorescein isothiocyanate (100 mg) and triethylamine (100 mg) in 40 mL of dichloromethane, then dissolve acryloyl chloride (231 mg) in 5 mL of dichloromethane, then Add it dropwise to the above solution, stir at room temperature for 3 h, remove most (85-95%) of dichloromethane by rotary evaporation after the reaction is completed, purify the product through a column, and dry it in vacuum to obtain product 1;
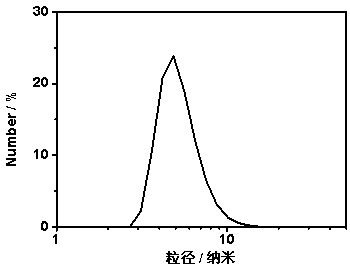
[0033] (2) Dissolve the product 1 (150 mg) synthesized in step (1) and carbon quantum dots (50 mg) prepared according to the prior art in dimethyl sulfoxide (10 mL) solution, react at room temperature for 24 hours, and react After the end, it was first precipitated with ether, then with methanol, centrifuged, and vacuum-dried to obtain product 2. Product 2 was then dissolved in water to a final concentration of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com