Application of CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) technique in coronary atherosclerotic heart diseases
A coronary atherosclerotic and sclerotic technology, applied in the field of medicine and biology, can solve the problems affecting the specific knockout of target genes, etc., and achieve the effects of high gene modification efficiency, large market application prospects, and simple operation techniques
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1 Primer Design
[0019] 1. Construct the in vitro Cas9 expression vector, named pGEM-Cas9, which was synthesized by Inwin Biotechnology, and its sequence is a commonly used sequence in the field.
[0020] 2. Construction and preparation of vectors for transcribing the sgRNA backbone coding sequence in vitro.
[0021] The sgRNA is designed for the mmu-miR-8086 gene, and the forward oligonucleotide sequence and the reverse oligonucleotide sequence can complement each other to form a double-stranded DNA fragment with sticky ends:
[0022] F: CACCGgcacagccttggtgtctctagtcc
[0023] R: CggactagagacaccaaggctgtgcCAAA.
Embodiment 2
[0024] Embodiment 2, construct the sgRNA expression vector of mmu-miR-8086 gene
[0025] 1. Synthesis of DNA Inserts
[0026] (1) Synthesize the forward and reverse oligonucleotide sequences designed above
[0027] The oligonucleotide sequence can be specifically synthesized by a commercial company (Shanghai Sangon Co., Ltd.) based on the provided sequence.
[0028] The corresponding forward and reverse oligonucleotide sequences are annealed and annealed to form double-stranded DNA fragments with cohesive ends.
[0029] The reaction system (20μL) is as follows:
[0030] Forward oligonucleotide (10 μM): 1 μL
[0031] Reverse oligonucleotide (10 μM): 1 μL
[0032] 10×PCR buffer: 2μL
[0033] wxya 2 O: 16 μL
[0034] Put the above reaction system into the PCR machine, and carry out the reaction according to the following procedure.
[0035] Reaction procedure:
[0036] 95℃, 5min;
[0037] 80℃, 5min;
[0038] 70℃, 5min;
[0039] 59℃, 5min;
[0040] 50℃, 5min;
[0041] ...
Embodiment 3
[0063] Embodiment 3, preparation of transgenic mice
[0064] 1) The CRISPR / Cas9 injection system for mice is as follows:
[0065] pGEM-Cas9
50ng / μl
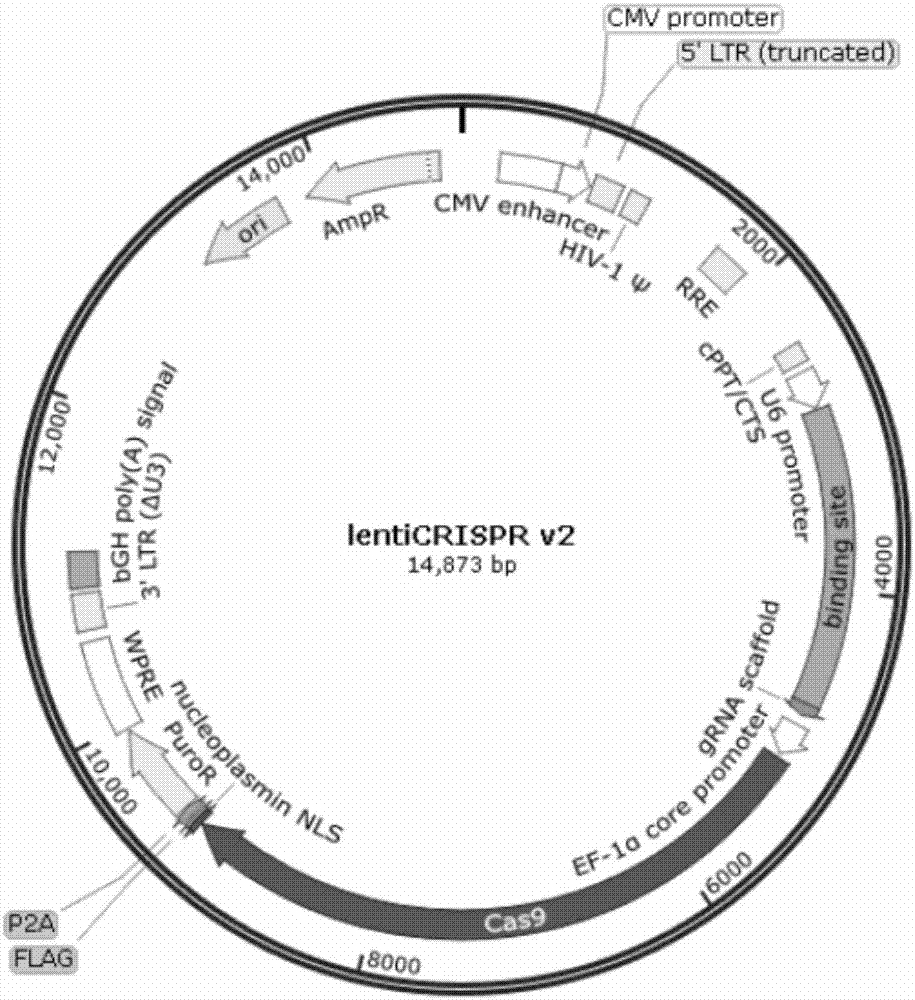
lentiCRISPR v2-sgRNA
10ng / μl
Mixed total volume
20μl
[0066] 2) injection
[0067] Use Eppendorf2xTransferManNK2 microinjector to draw 2 μl of the mixture in step 1) and inject 60 fertilized eggs. Subsequent conception.
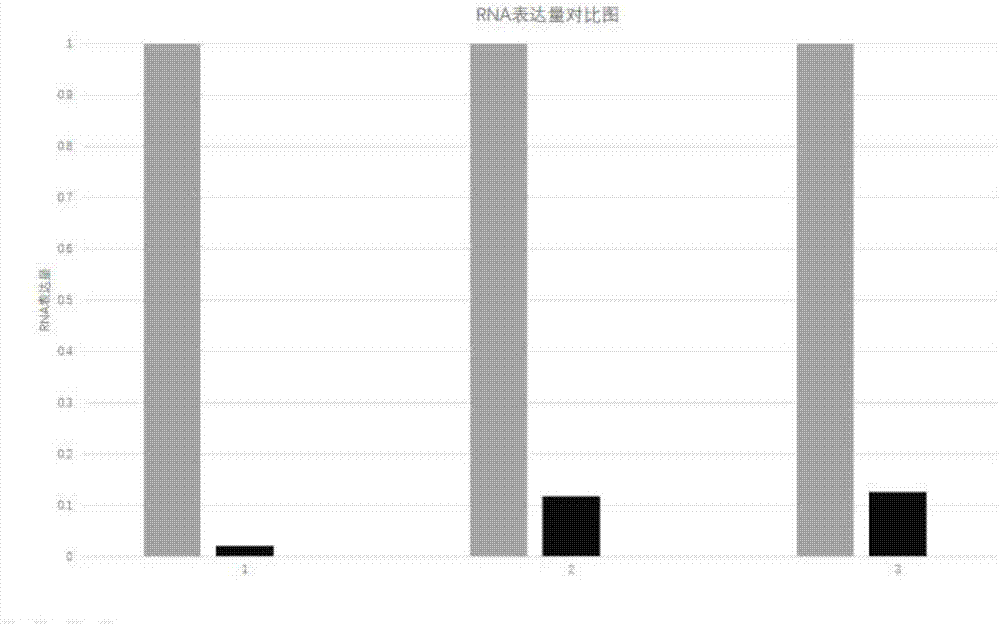
[0068] Five days after the mice were born, the mouse nails were clipped to extract genomic DNA. The gene expression levels of miR-8086, Card3 and SHPS-1 were identified by PCR, and 30 mice without gene knockout were used as controls. Such as figure 2 As shown, compared with the control, the gene expression levels of miR-8086, Card3 and SHPS-1 in the mice were reduced to 0.2%, 11.7%, and 12.5%, respectively, compared with the control. This fully shows that miR-8086 in mice has been completely knocked out, and gene expression has been almost completely lost. Correspon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com