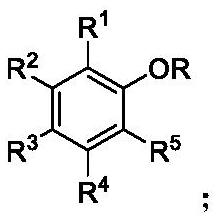
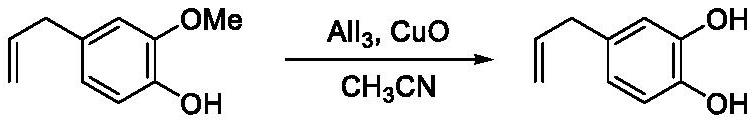A kind of ether bond cleavage method of phenyl alkyl ether
A technology of phenyl alkyl ether and ether bond, which is applied in the field of intermediate synthesis of medicines and chemical raw materials, can solve the problems of physical injury of operators, air pollution, heavy pyridine odor, etc., and achieves wide application scope and low environmental pollution. , the effect of small air pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
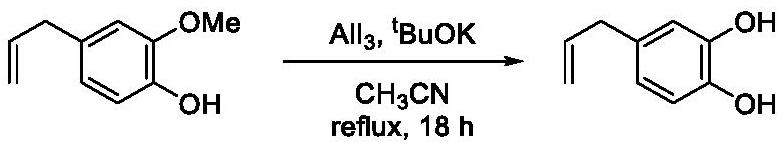
[0030] Embodiment 1 (eugenol demethylation)
[0031]
[0032] Add aluminum triiodide (2.242g, 5.5mmol), acetonitrile (40ml), potassium tert-butoxide (1.237g, 11.0mmol) and eugenol (0.819g, 5.0mmol) to a 100ml eggplant-shaped flask, heat to 80 ℃, stop stirring after 18 hours of reaction, cool to room temperature, add 2mol / L dilute hydrochloric acid (10ml) into the eggplant-shaped bottle to acidify, extract with ethyl acetate (50ml×3), combine the organic phases, and first use thiosulfuric acid Wash with saturated sodium aqueous solution (10ml), then with saturated brine (10ml), dry over anhydrous magnesium sulfate, filter, and the filtrate is evaporated to dryness with a rotary evaporator, and the residue is subjected to flash column chromatography (eluent is ethyl acetate / Petroleum ether=1:4, volume ratio) purification to obtain 0.733g 4-allyl catechol crude product, take the 4-allyl catechol crude product (0.709g) and sublimate under reduced pressure with an oil pump to o...
Embodiment 2
[0035] Embodiment 2 (eugenol demethylation)
[0036]
[0037] CuO (5.966g) was used to replace potassium tert-butoxide, and the other conditions were the same as in Example 1 to obtain 4-allyl catechol (yield 88%).
Embodiment 3
[0038] Embodiment 3 (eugenol demethylation)
[0039]
[0040] CaO (4.200g) was used to replace CuO, and the remaining conditions were consistent with Example 2 to obtain 4-allyl catechol (yield 95%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com