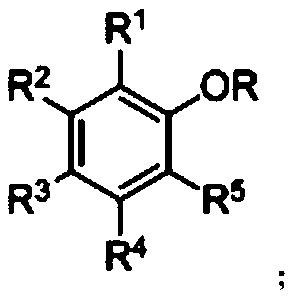
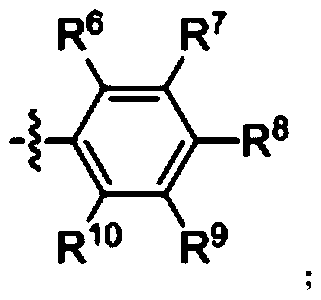
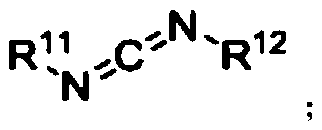
A kind of ether bond breaking method of phenyl alkyl ether
A technology of phenyl alkyl ether and ether bond, which is applied in the field of intermediate synthesis of pharmaceuticals and chemical raw materials, can solve the problem of low yield of eugenol demethylation reaction, and achieve the effect of wide application range and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 (demethylation of eugenol)
[0035]
[0036] Aluminium triiodide (2.247g), acetonitrile (40ml), DCC (0.618g) and eugenol (0.818g) were added to a 100ml eggplant-shaped flask, heated to 80°C, and the stirring was stopped after 18 hours of reaction, and cooled to After room temperature, 2mol / L dilute hydrochloric acid (50ml) was added to the eggplant-shaped flask to acidify, extracted with ethyl acetate (50ml×3), the organic phases were combined, washed with saturated aqueous sodium thiosulfate solution (10ml), and then with saturated aqueous solution (10ml). Washed with brine (10ml), dried over anhydrous magnesium sulfate, filtered, the filtrate was evaporated to dryness with a rotary evaporator, and the residue was purified by flash column chromatography (eluent: ethyl acetate / petroleum ether=1:4, volume ratio) , to obtain 0.721 g of 4-allyl catechol (white waxy solid, yield 96%).
[0037] 1 H NMR (400MHz, CDCl 3 )δ6.80(d,J=8Hz,1H),6.72(d,J=2Hz,1H),6.63(...
Embodiment 2
[0039] Embodiment 2 (eugenol demethylation)
[0040] Add aluminum triiodide (2.252g), acetonitrile (40ml), N,N'-dicyclohexylcarbodiimide DCC (1.036g) and eugenol (0.818g) respectively in a 100ml eggplant-shaped bottle, and heat to 80°C, stop stirring after 18 hours of reaction, cool to room temperature, add 2mol / L dilute hydrochloric acid (50ml) into the eggplant-shaped bottle to acidify, extract with ethyl acetate (50ml×3), combine the organic phases, and first use thio Wash with saturated aqueous sodium sulfate (10ml), then with saturated brine (10ml), dry over anhydrous magnesium sulfate, filter, and evaporate the filtrate to dryness with a rotary evaporator, and the residue is subjected to flash column chromatography (eluent is ethyl acetate / petroleum ether=1:4, volume ratio) to obtain 0.723 g of 4-allyl catechol (white waxy solid, yield 96%).
Embodiment 3
[0041] Embodiment 3 (eugenol demethylation)
[0042] Add aluminum triiodide (2.247g), acetonitrile (40ml), DCC (1.549g) and eugenol (0.818g) respectively in a 100ml eggplant-shaped bottle, heat to 80 ° C, stop stirring after 18 hours of reaction, cool to After room temperature, add 2mol / L dilute hydrochloric acid (50ml) into the eggplant-shaped bottle to acidify, extract with ethyl acetate (50ml×3), combine the organic phases, wash with saturated aqueous sodium thiosulfate (10ml), and then wash with saturated Wash with brine (10ml), dry over anhydrous magnesium sulfate, filter, evaporate the filtrate to dryness with a rotary evaporator, and purify the residue by flash column chromatography (eluent: ethyl acetate / petroleum ether=1:4, volume ratio) , to obtain 0.684 g of 4-allyl catechol (white waxy solid, yield 91%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com