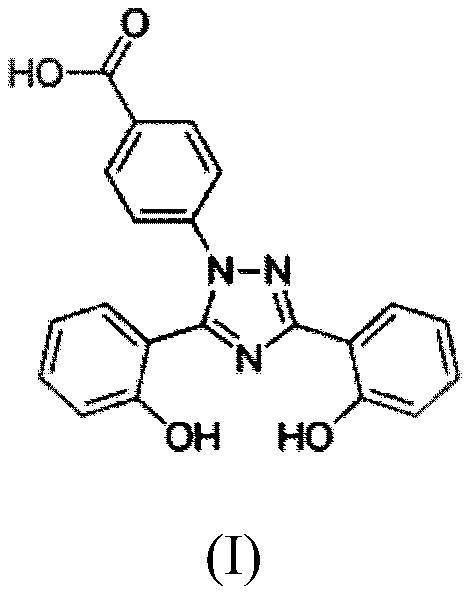
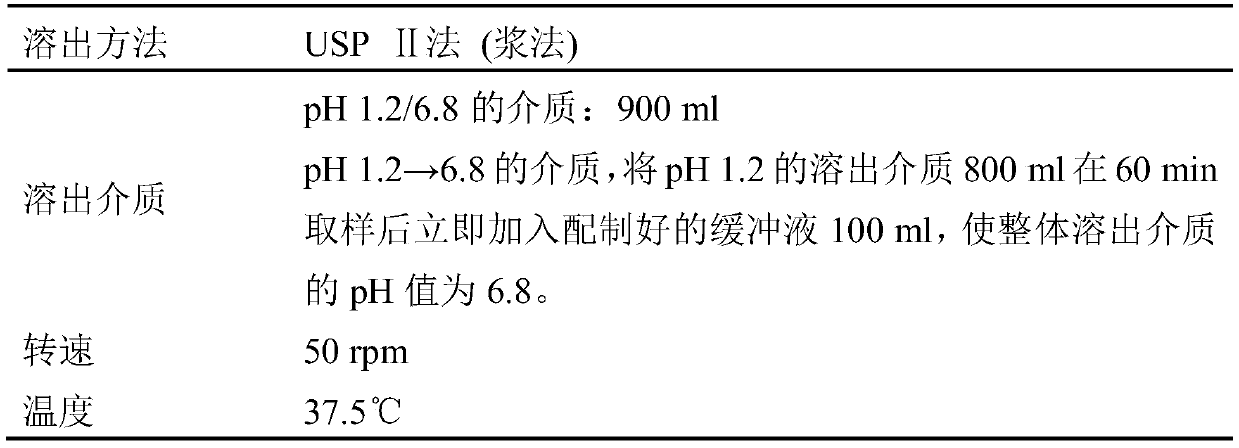
A kind of deferasirox pharmaceutical composition and its pharmaceutical preparation, preparation method and application
A technology for deferasirox and pharmaceutical preparations, which can be applied in the directions of drug combinations, pharmaceutical formulations, antidote and the like, and can solve problems such as inconvenience for patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
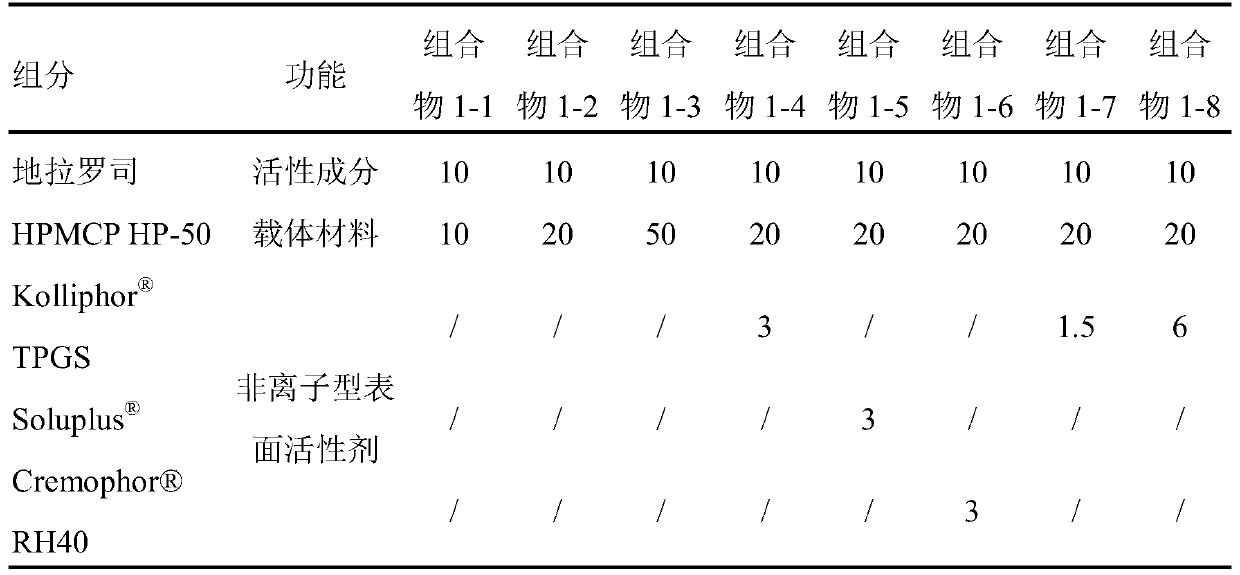
[0102] Embodiment 1 deferasirox-HPMCP pharmaceutical composition / physical mixture
[0103] 1. Preparation:
[0104] According to the specific composition and dosage in Table 1-1, deferasirox and carrier material HPMCP (being specifically HP-50) and optional nonionic surfactant (such as TPGS, RH40) are respectively added in the mixer and mixed evenly, and then fed into the feed hopper of the co-rotating twin-screw extruder (Omicron12 of India Steer Company); or by the specific composition and consumption in Table 1-1, the above-mentioned ingredients are directly fed into Feed into the hopper of the co-rotating twin-screw extruder. Set the melting temperature of the co-rotating twin-screw extruder to about 140°C, the screw speed to about 150rpm, and the L / D to 25. The extruded product is cooled, crushed, and sieved to obtain a solid powder, namely this The inventive deferasirox pharmaceutical composition (composition for short, such as composition 1-1, composition 1-4, etc....
Embodiment 2
[0130] The pharmacokinetic research of embodiment 2 deferasirox-HPMCP pharmaceutical composition
[0131] 1. Test preparation
[0132] Test preparation: deferasirox-HPMCP tablet prepared according to the prescription and process of pharmaceutical composition 1-2 and external pharmaceutical excipient composition 2-1 in Example 1, the specification is 500 mg.
[0133] Reference preparation: deferasirox dispersible tablets developed and marketed by Novartis The specification is 500mg, and the inactive ingredients include lactose monohydrate, crospovidone, povidone K30, sodium lauryl sulfate, microcrystalline cellulose, silicon dioxide, and magnesium stearate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com