A relay-recognition fluorescent probe and its synthesis and application
A technology of fluorescent probes and probes, which is applied in the direction of fluorescence/phosphorescence, luminescent materials, and material analysis through optical means, which can solve the problems of expensive instruments, cumbersome operations, etc., and achieve low detection limits, high sensitivity, and good results. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: the synthesis of intermediate compound 2
[0045]
[0046] (1) Synthesis of compound 3
[0047] A mixture of 8-hydroxy-2-methylquinoline (1.6 g, 10.05 mmol), ethyl bromoacetate (1.7 g, 10.18 mmol) and anhydrous potassium carbonate (5 g, 36.18 mmol) in acetone (20 mL) was heated Reflux for 24 hours. After cooling, the mixture was filtered and the resulting filtrate was evaporated to dryness to give a crude residue. The final product was purified by chromatography (eluent, dichloromethane / ethyl acetate=10:1) to obtain 1.98 g of light yellow oily product, namely compound 3.
[0048] (2) Synthesis of Compound 2
[0049] At 65 °C, 0.75 g of SeO2 was added to a solution of 3 (1.5 g, 6 mmol) in 1,4-dioxane (20 mL), and the temperature of the above mixture was raised to 80 °C. After reacting for 2 hours, it was cooled to room temperature. The precipitate was filtered off. The organic phase was concentrated in vacuo. The final product was obtained as a ye...
Embodiment 2
[0051] Embodiment 2: Synthesis of fluorescent probes based on quinoline structure
[0052]
[0053] Add 0.58g (4mmol) 5-chloro-2-hydroxyaniline and 1.04g compound 2 (4mmol) to a 100mL round-bottomed flask, then add 20mL ethanol solvent to dissolve, add 3 drops of acetic acid and 5 mg of ZnO as a catalyst, 50 ° C The reaction was stirred for 4 hours. The reaction process was monitored by TLC (developing solvent: petroleum ether / ethyl acetate=1:2), and the reaction of compound 2 was the end point. The crude product was recrystallized from ethanol and purified by column chromatography (eluent: petroleum ether / dichloromethane=0.5:1). Finally, 1.13 g of a light yellow solid, namely probe 1, was obtained with a yield of 73.8%.
Embodiment 3
[0054] Embodiment 3: Synthesis of fluorescent probes based on quinoline structure
[0055] Add 1.75g (12mmol) of 5-chloro-2-hydroxyaniline and 3.12g of compound 2 (12mmol) into a 100mL round bottom flask, then add 40mL of ethanol solvent to dissolve, add 10 drops of acetic acid and 20 mg of ZnO as a catalyst, and then heat at 80°C The reaction was stirred for 8 hours. The reaction process was monitored by TLC (developing solvent: petroleum ether / ethyl acetate=1:2), and the reaction of compound 2 was the end point. The crude product was recrystallized from ethanol and purified by column chromatography (eluent: petroleum ether / dichloromethane=0.5:1). Finally, 3.25 g of a light yellow solid, namely probe 1, was obtained with a yield of 70.8%.
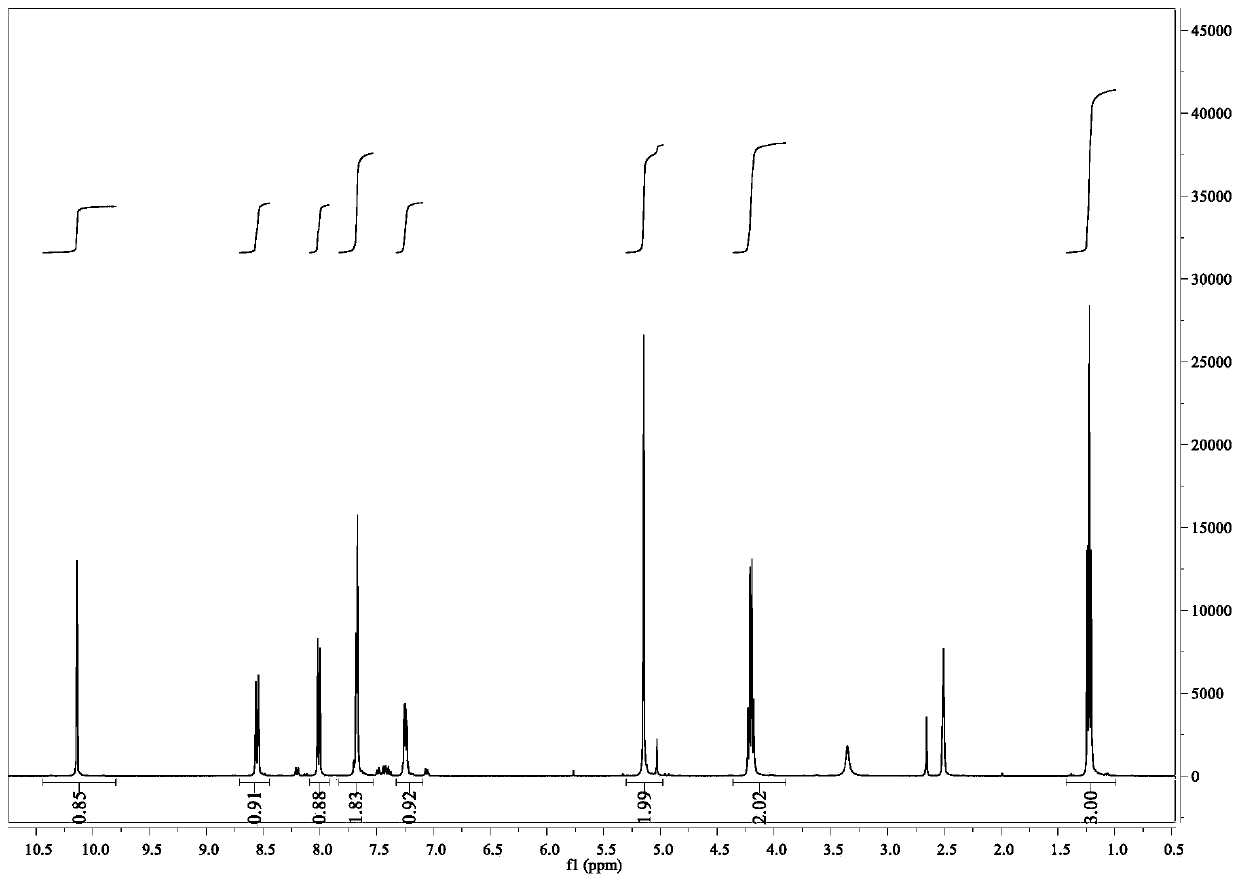
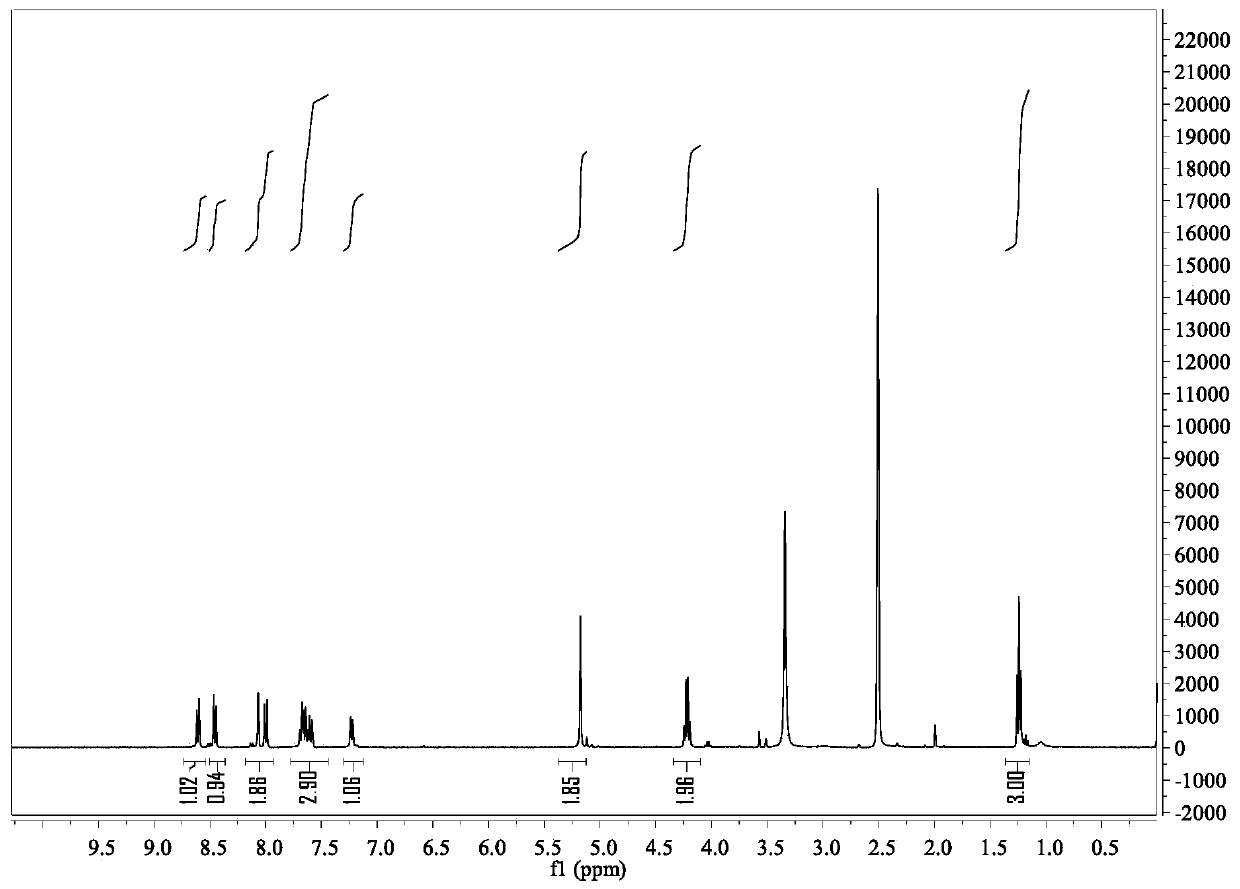
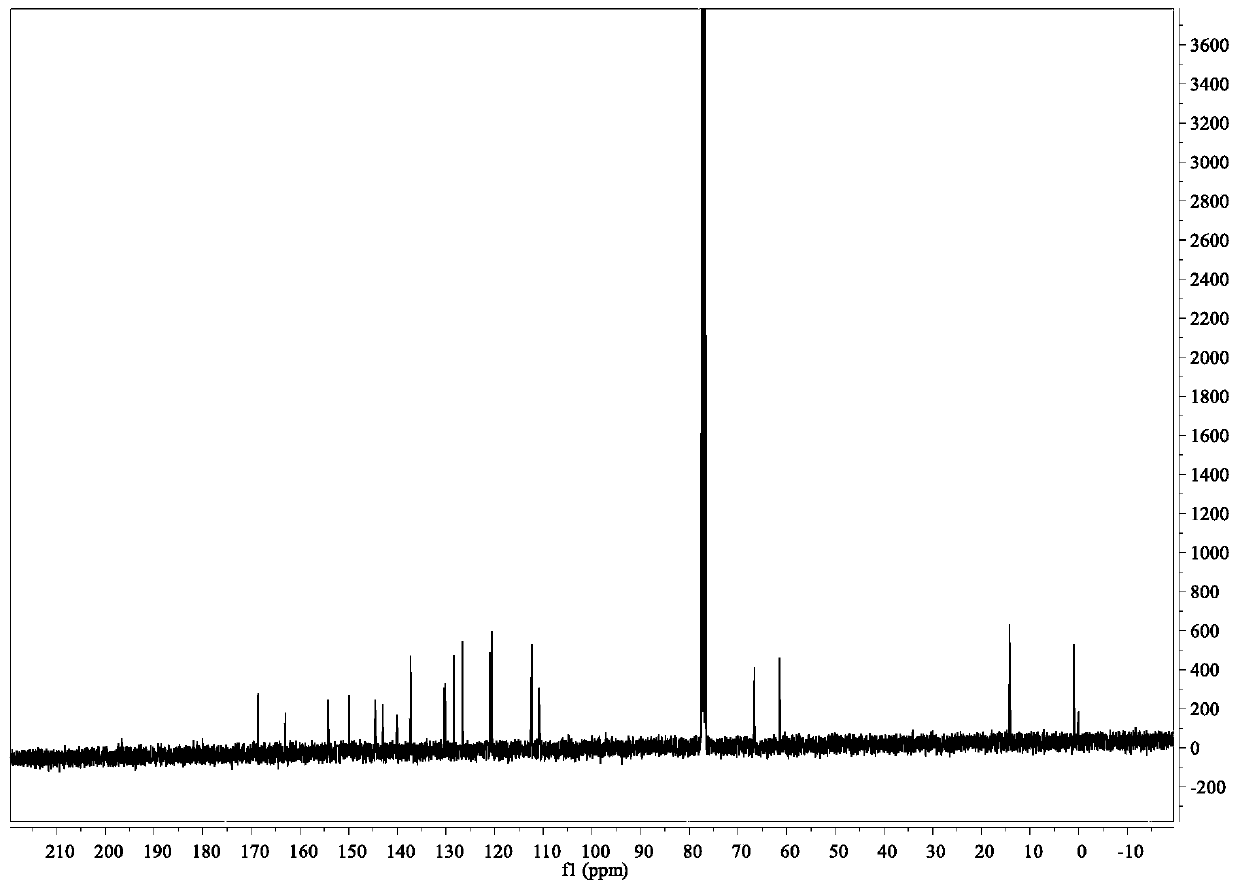
[0056] Its NMR image is figure 2 and 3 Shown: 1 H NMR (400MHz, DMSO-d6) δ8.6(dd,1H),8.45(dd,1H),8.05(dd,1H),8.0(dd,1H),7.82–7.56(m,3H),7.23( dd,1H),5.18(s,2H),4.22(q,2H),1.24(t,3H); 13 C NMR (101MHz, DMSO-d6) δ169.15, 163.40, 154...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com