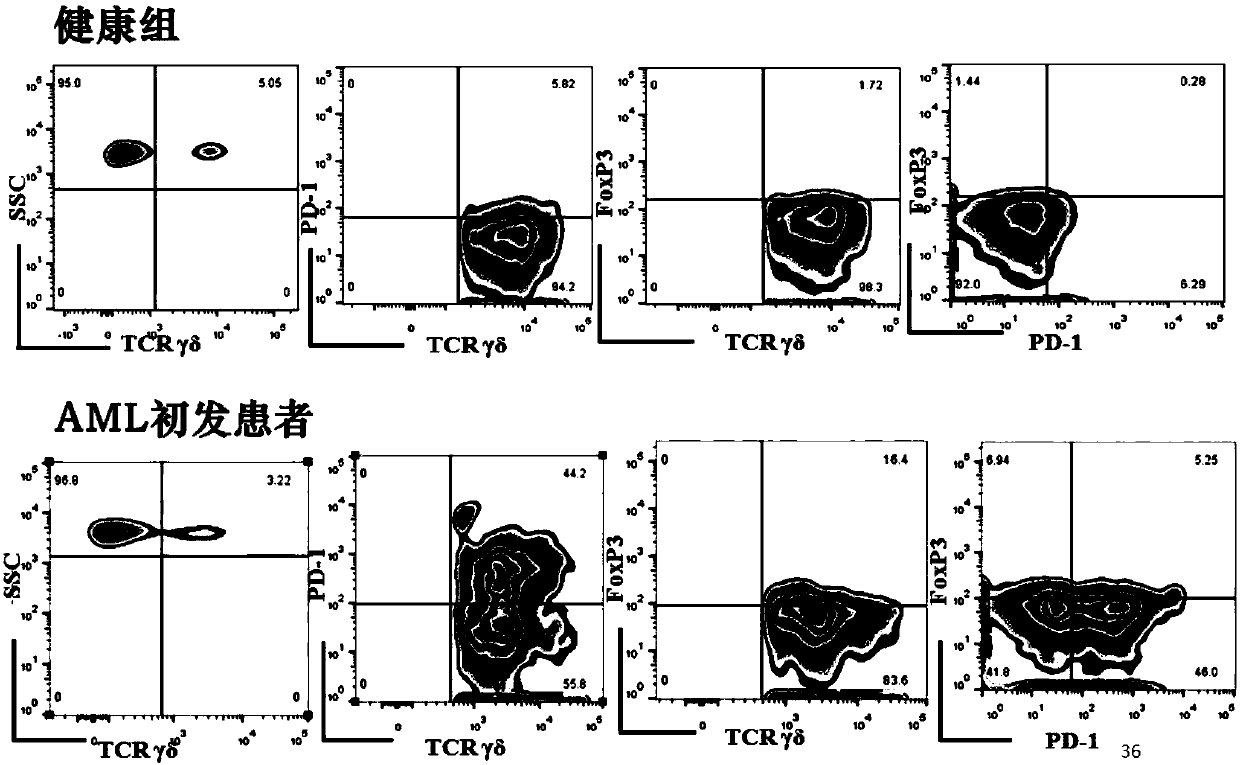
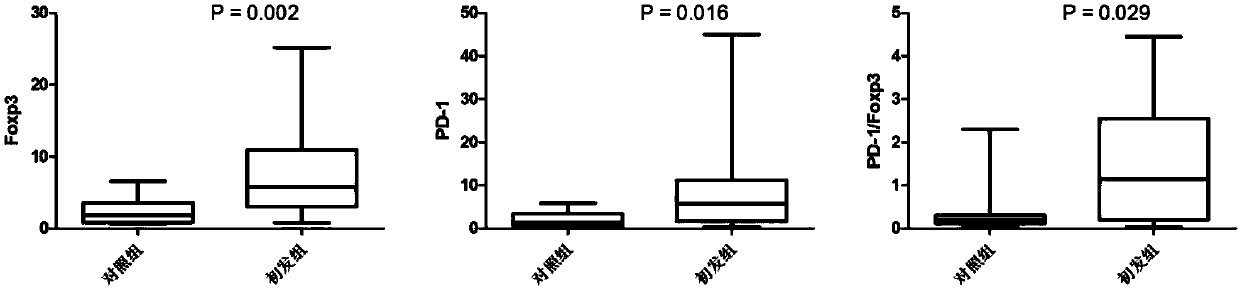
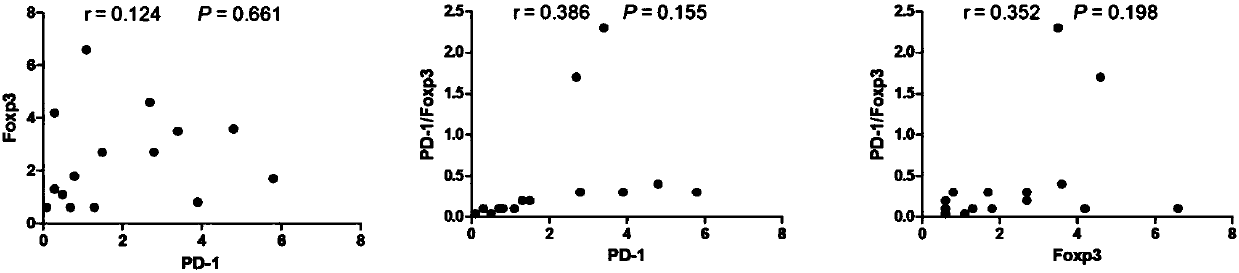
Application of novel gamma delta T cell to preparation of kit for evaluating curative effect of AML (acute myeloid leukemia)
A kit and curative effect technology, applied in the field of biomedicine, can solve the problems of recurrence or drug resistance, no treatment method, no γδTreg cell research, etc., and achieve a broad clinical effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] (1) On the premise of signing the informed consent form with the patient, all samples were taken from fasting venous blood anticoagulated with heparin in the morning. Peripheral blood samples were collected from 14 patients with initial AML, 5 patients with relapse, and 4 patients with remission. At the same time, 15 healthy adult samples were collected, and this part of the research protocol has been approved by the ethics committee of the unit. At the same time, clinical data such as clinical curative effect of AML patients were collected (as shown in Table 1).
[0073] (2) Separation of peripheral blood mononuclear cells. Take 4mL of lymphocyte separation medium (Ficoll, density 1.077) and add it into a 15mL centrifuge tube, spread the diluted anticoagulated peripheral blood sample suspension on the separation medium, and centrifuge at 1500rpm for 15 minutes in a horizontal centrifuge. Aspirate the single nuclear layer in the middle and transfer it to another 15mL ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com