Patents
Literature
167 results about "FOXP3" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
FOXP3 (forkhead box P3), also known as scurfin, is a protein involved in immune system responses. A member of the FOX protein family, FOXP3 appears to function as a master regulator of the regulatory pathway in the development and function of regulatory T cells. Regulatory T cells generally turn the immune response down. In cancer, an excess of regulatory T cell activity can prevent the immune system from destroying cancer cells. In autoimmune disease, a deficiency of regulatory T cell activity can allow other autoimmune cells to attack the body's own tissues.
IL-2 derivative polypeptides
ActiveUS9206243B2Good treatment effectLow ability to stimulateAntibacterial agentsPeptide/protein ingredientsRegulatory T cellInfectious Disorder
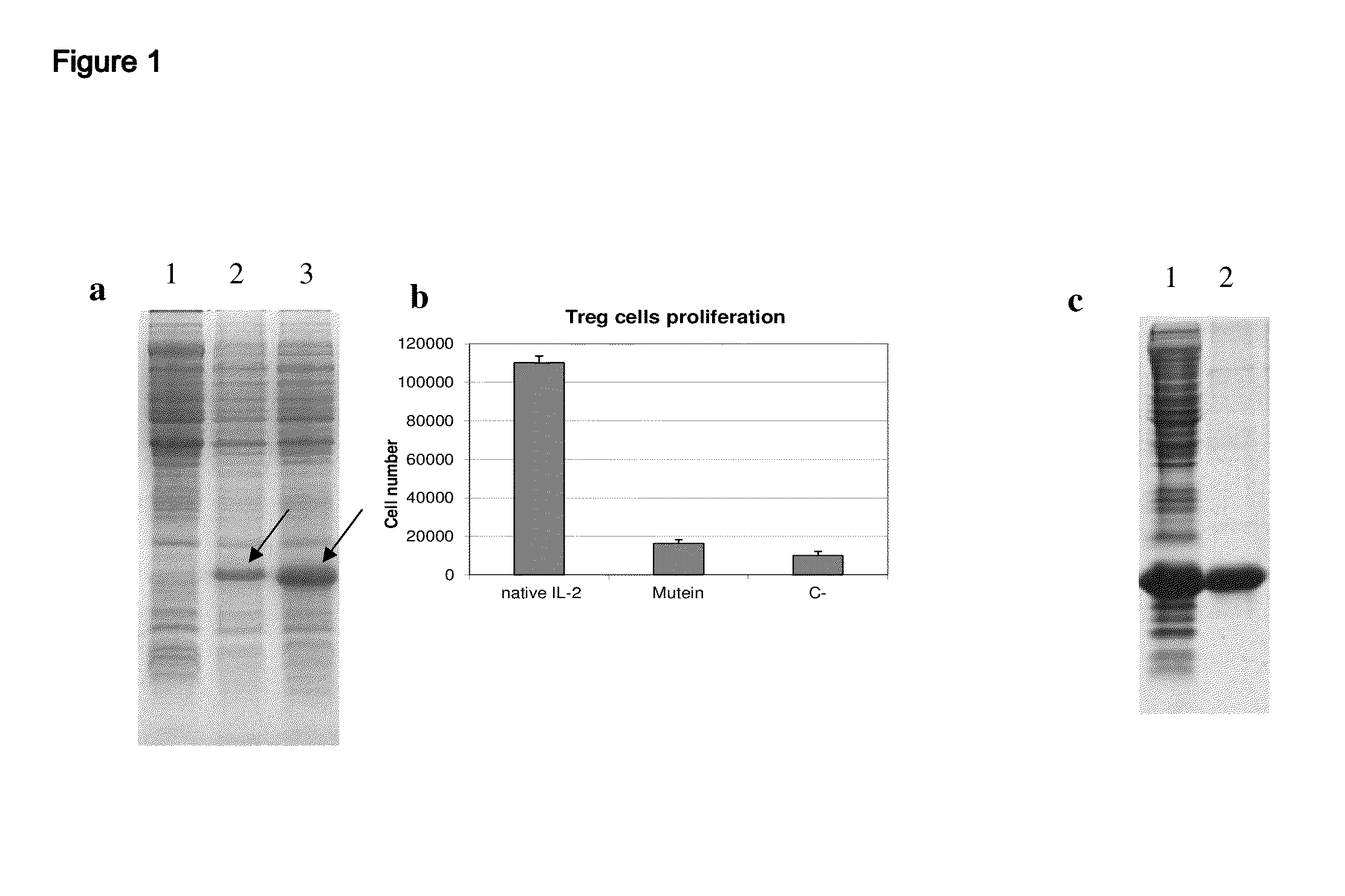
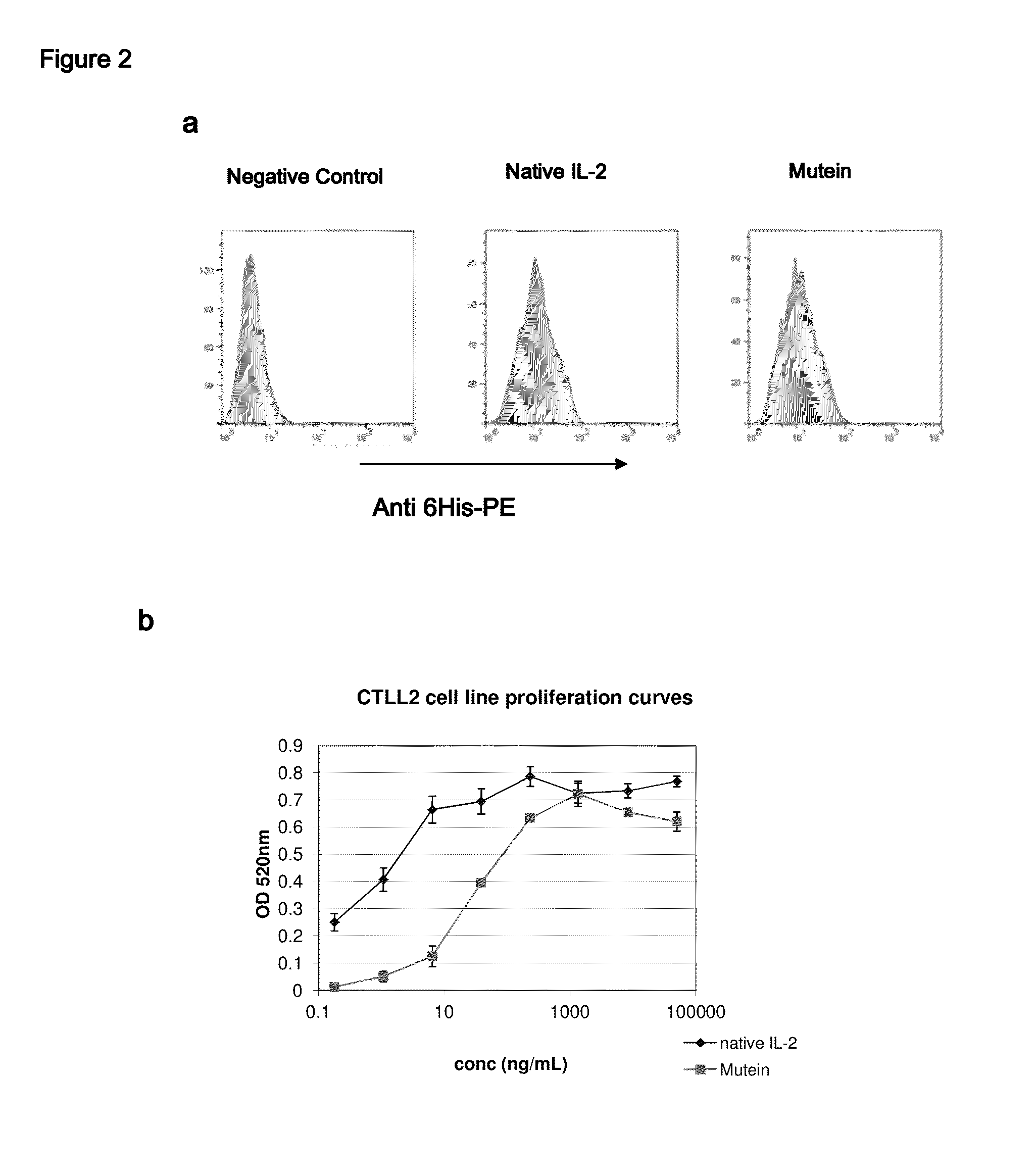
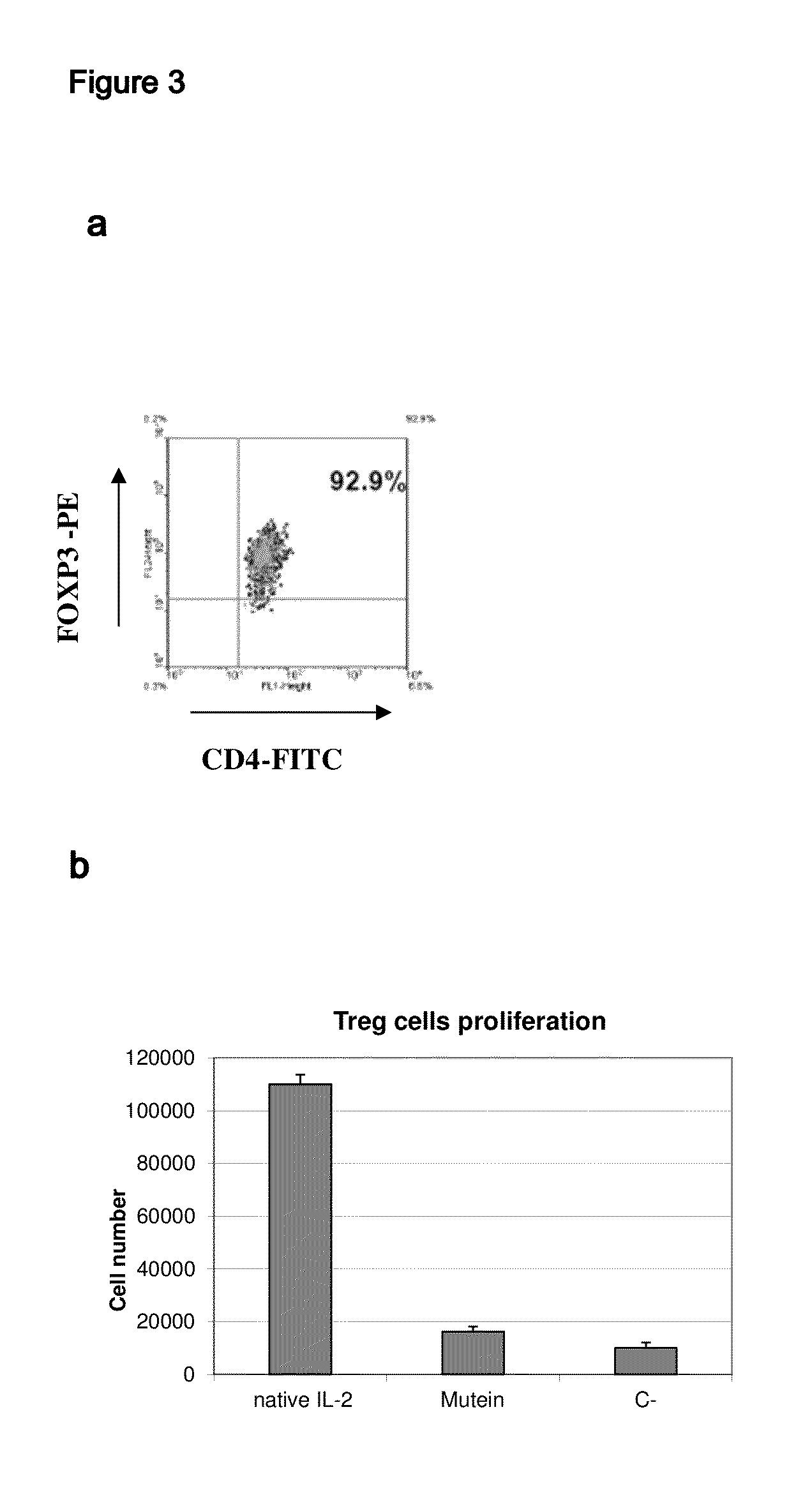
The present invention relates to polypeptides which share primary sequence with human IL-2, except for several amino acids that have been mutated. The mutations introduced substantially reduce the ability of these polypeptides to stimulate in vitro and in vivo regulatory T cells (T CD4+CD25+FoxP3+) and make them more effective in the therapy of murine transplantable tumors. Also includes therapeutic uses of these mutated variants, used alone or in combination with vaccines for the therapy of diseases such as cancer or infections where the activity of regulatory T cells (Tregs) is relevant. In another aspect the present invention relates to pharmaceutical compositions comprising as active principle the polypeptides disclosed. Finally, the present invention relates to the therapeutic use of the polypeptides and pharmaceutical compositions disclosed due to their modulating effect of the immune system on diseases like cancer and chronic infectious diseases.
Owner:CENT DE INMUNOLOGIA MOLECULAR CENT DE INMUNOLO
Methods of modulating the ox40 receptor to treat cancer
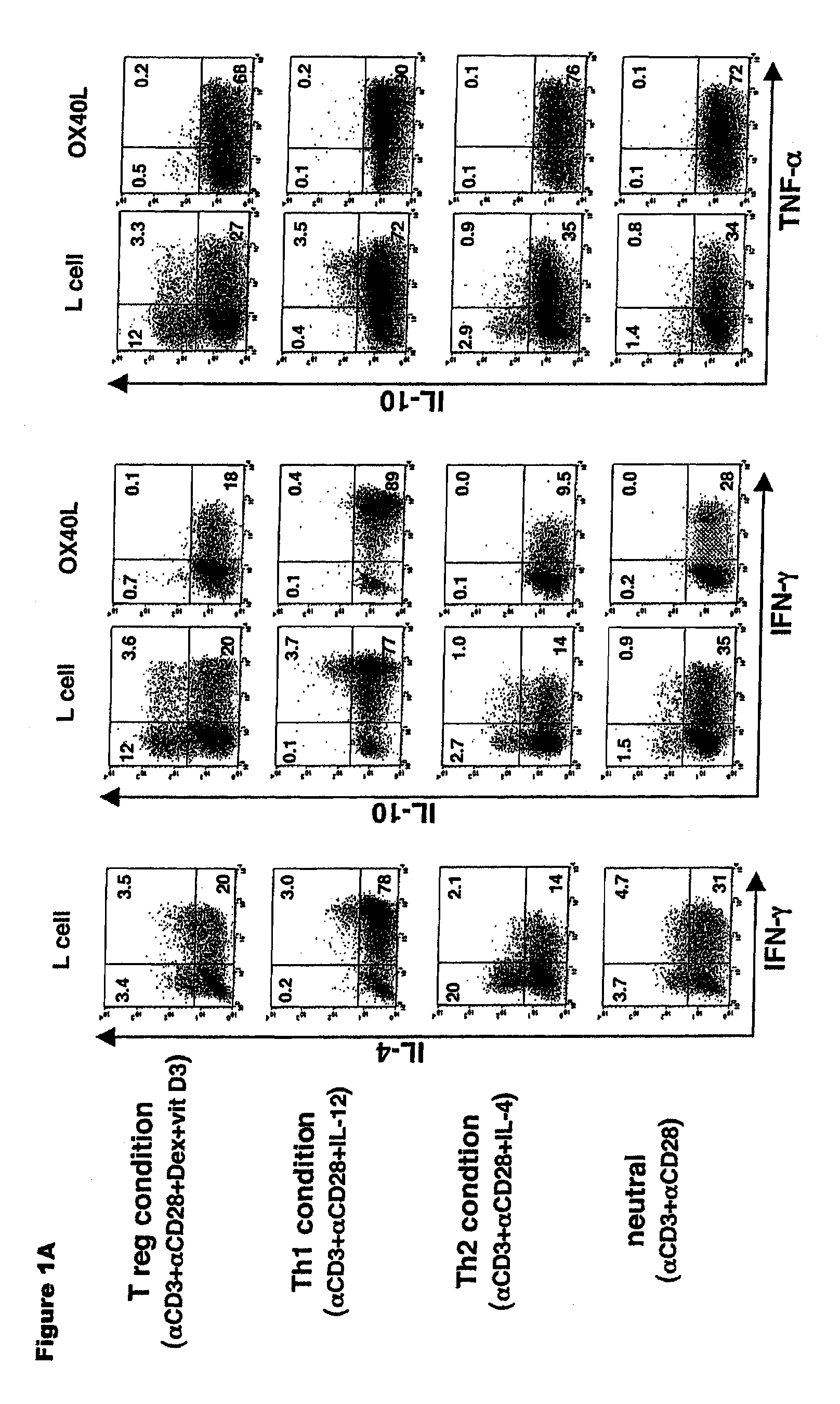
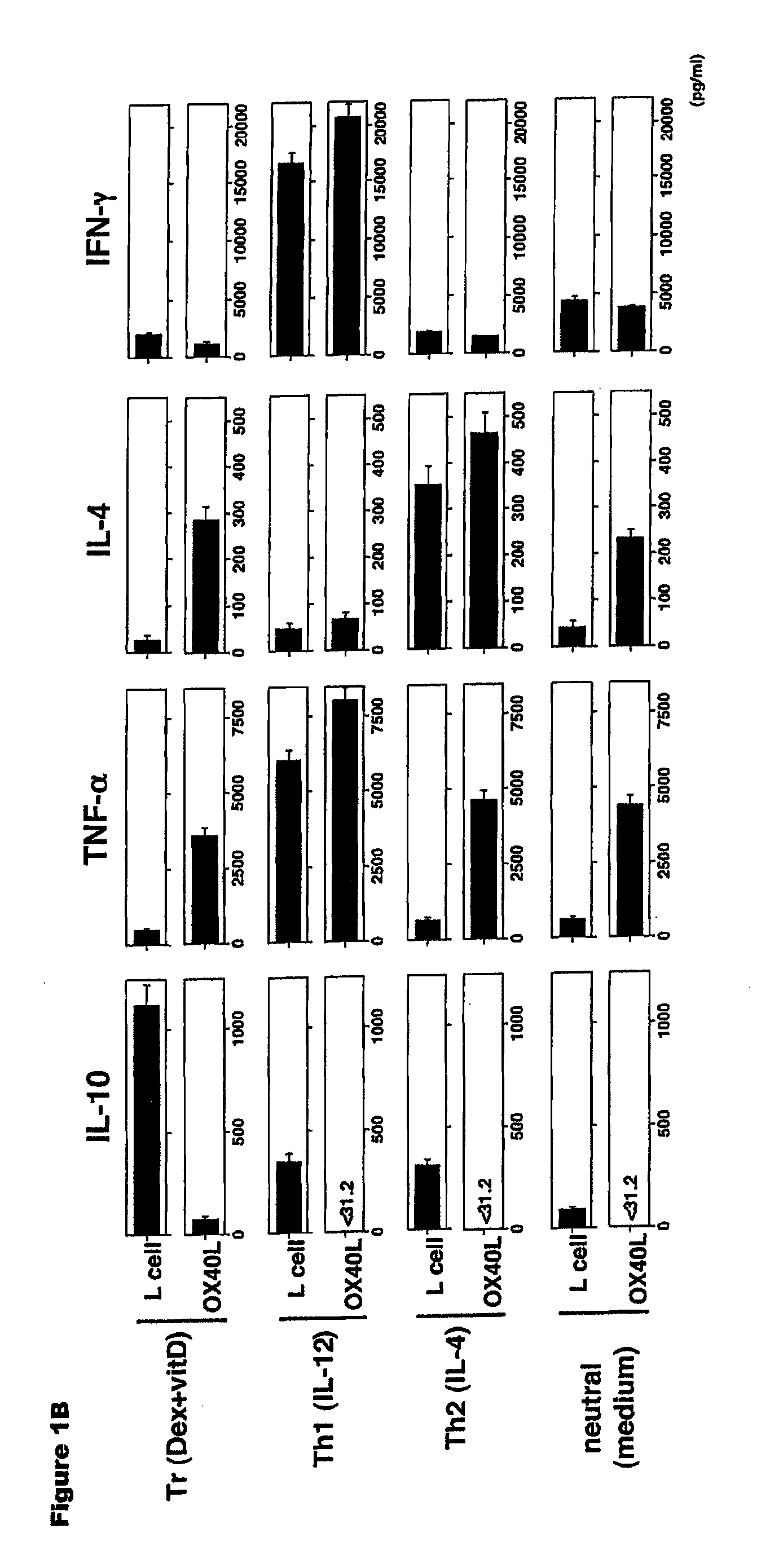
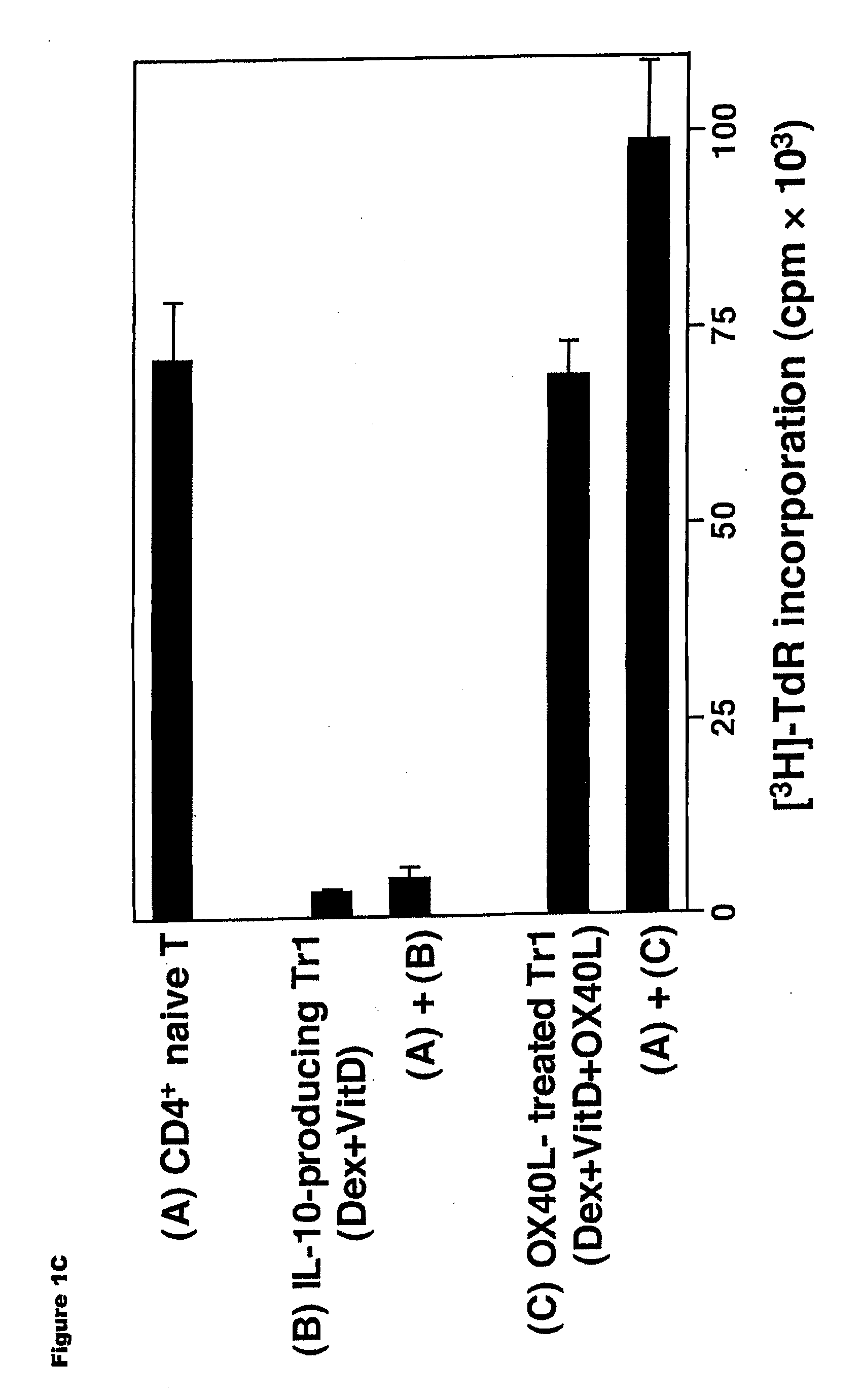
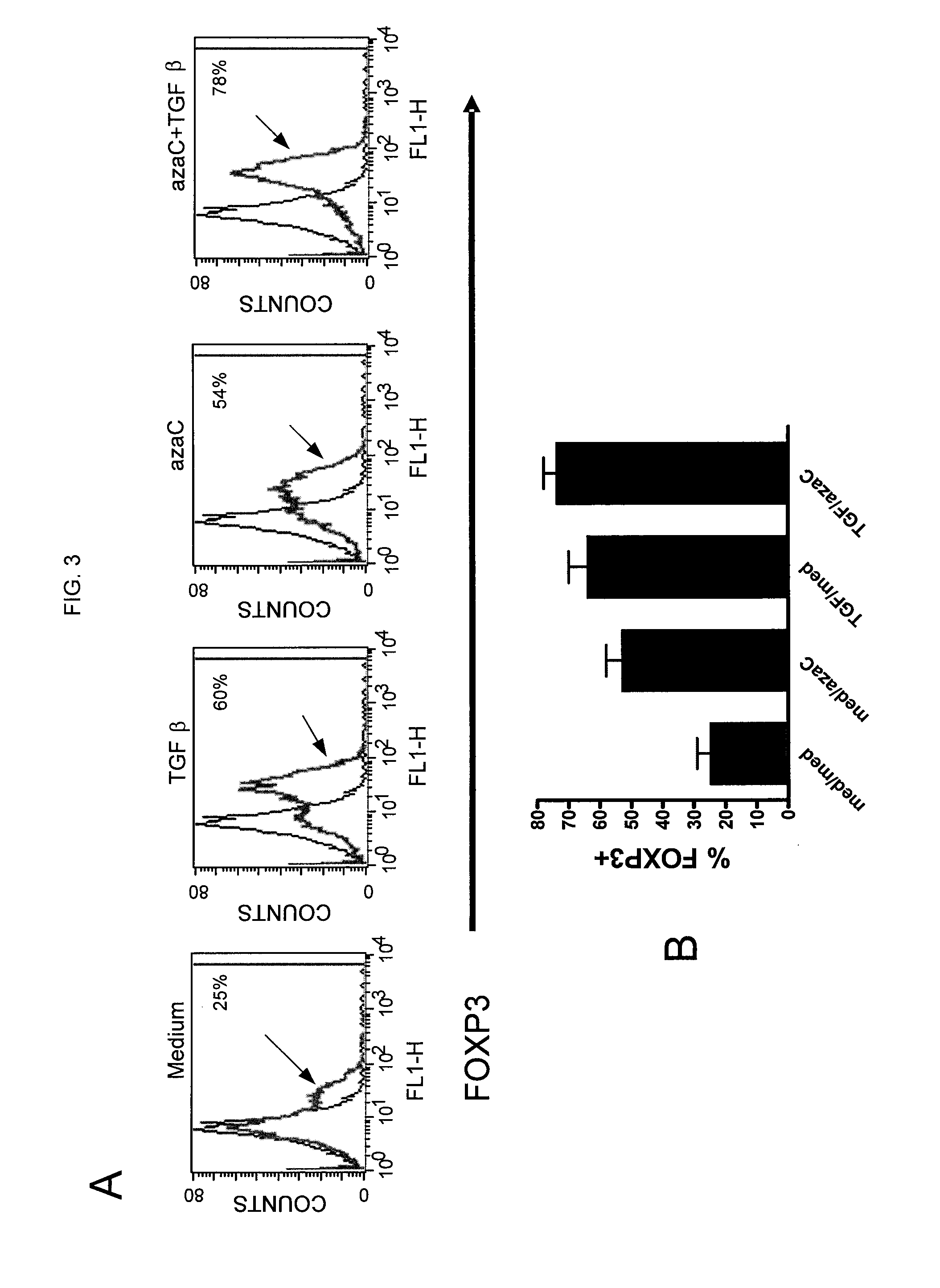
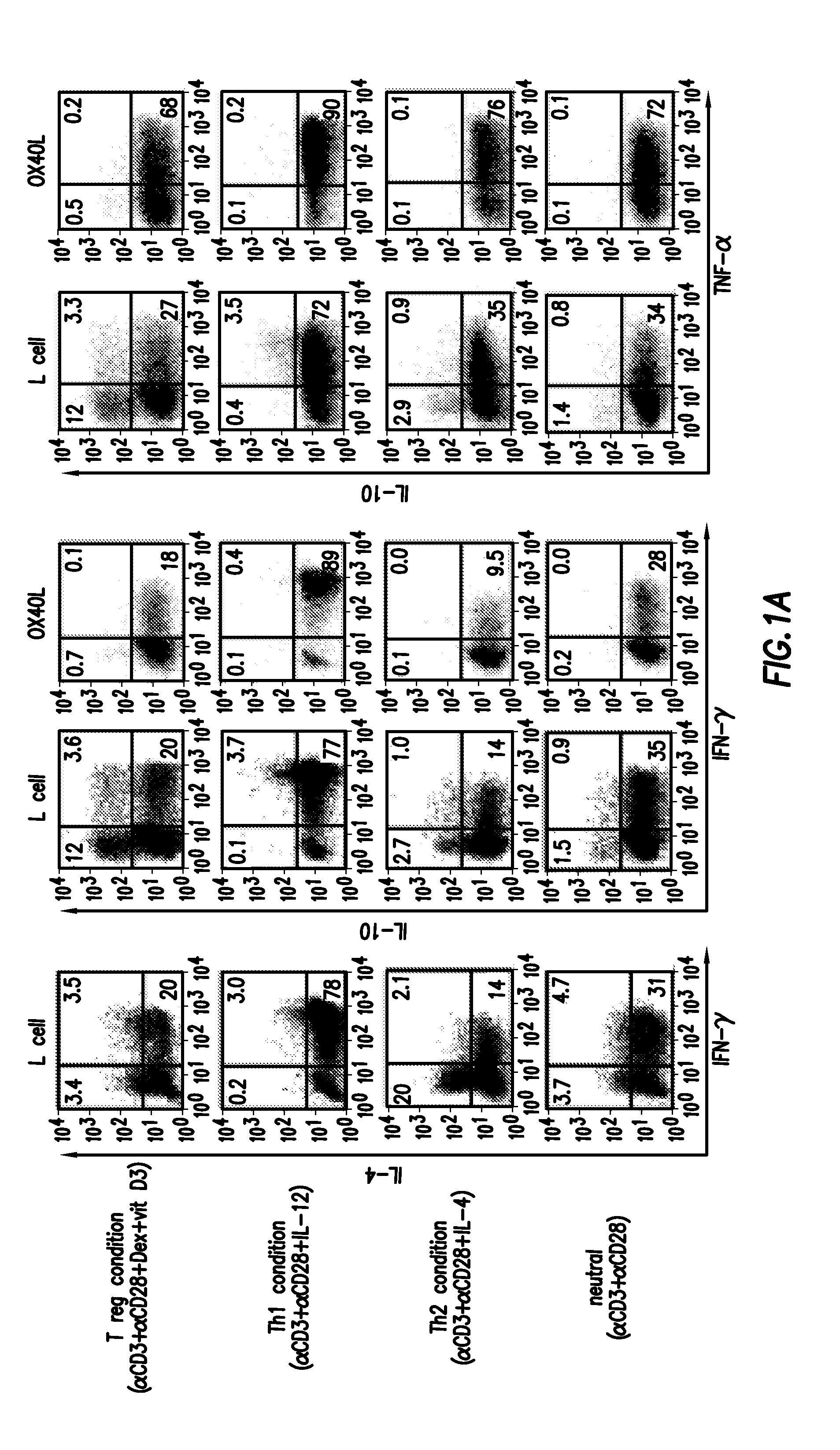
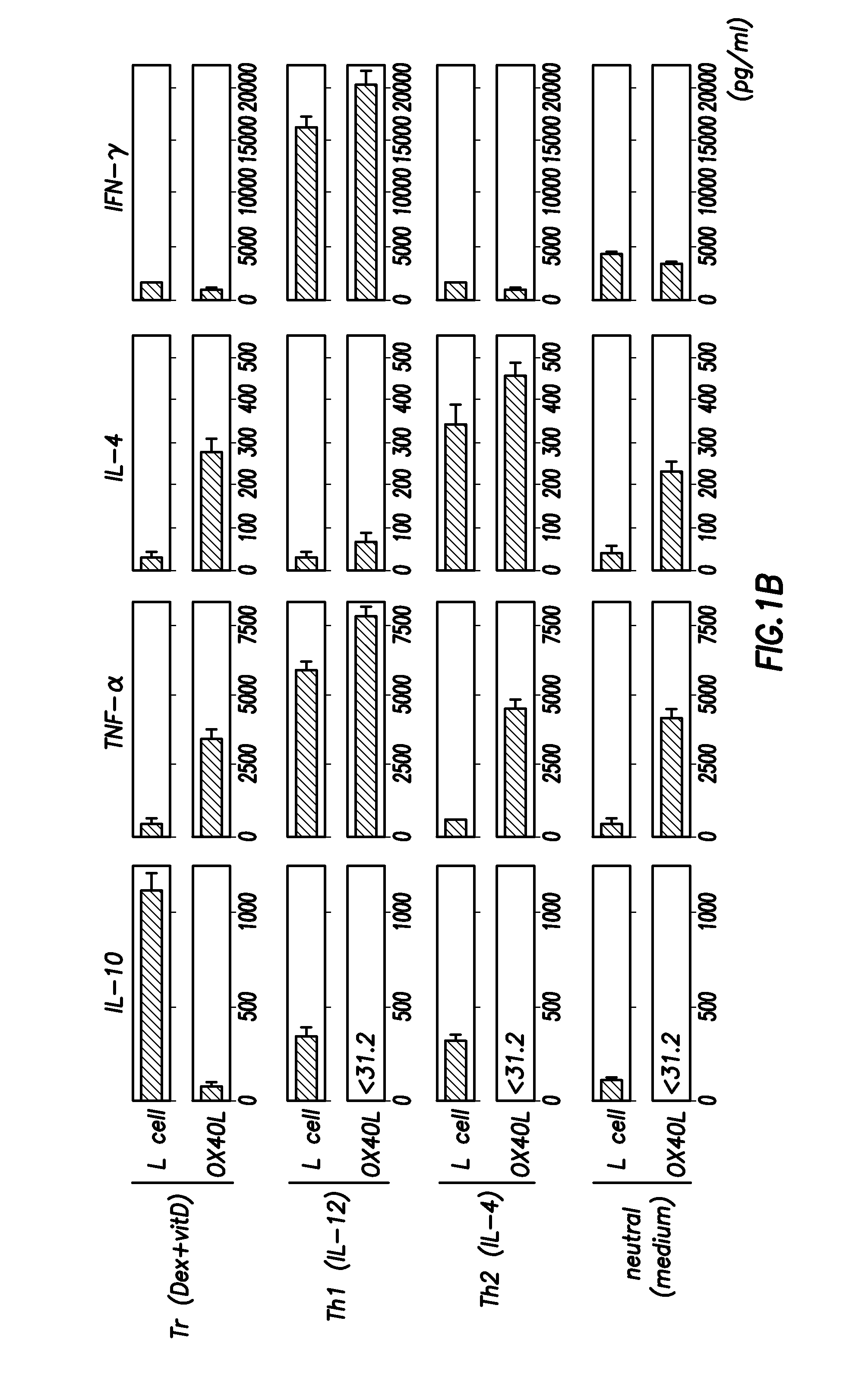
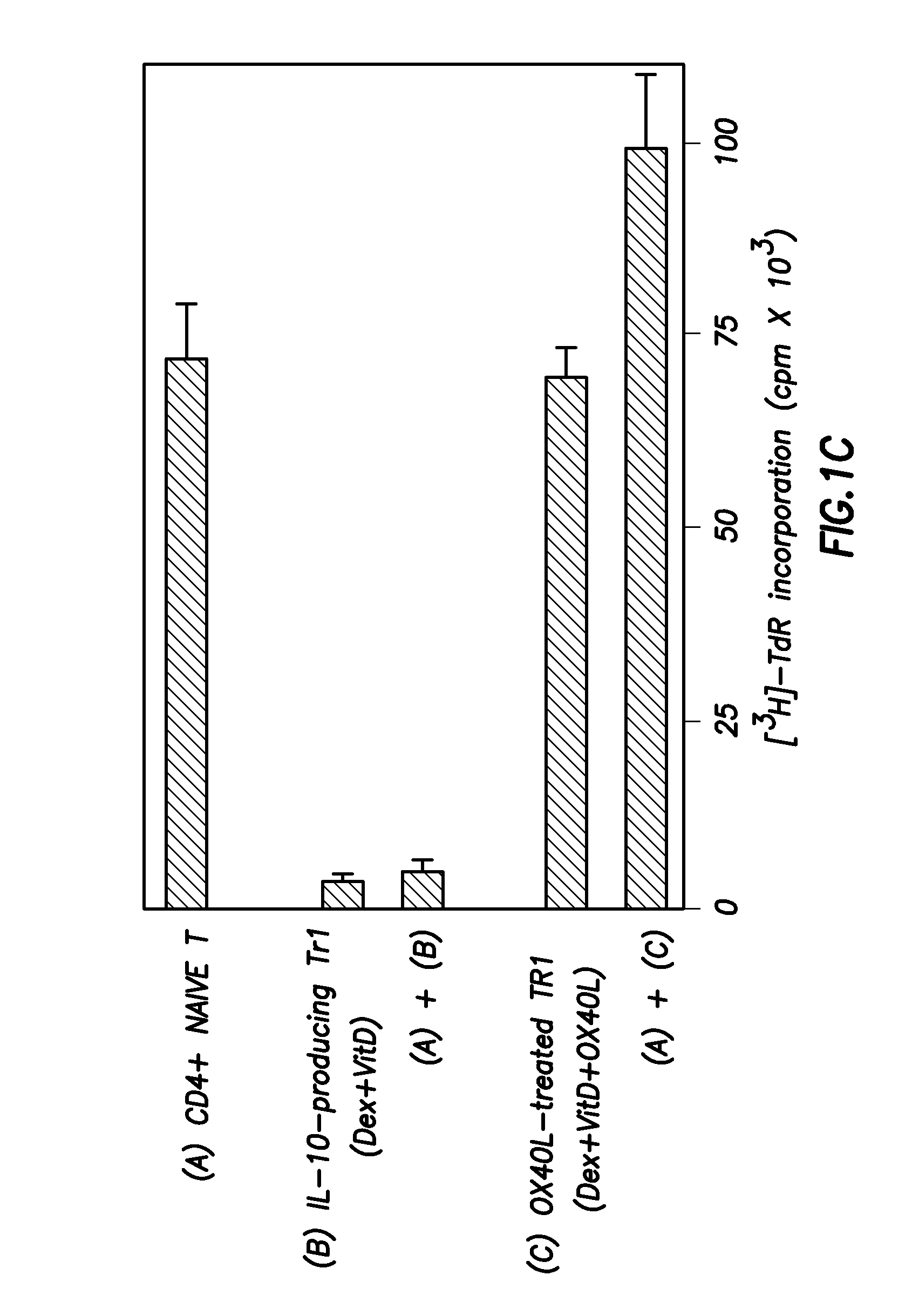
Numerous disease states, such as human allergic, autoimmune, and autoimmune diseases, and cancer, may be treated by targeting OX40 / OX4OL. OX4OL inhibits the generation of Tr1 cells from naïve and memory CD4+ T cells. This unique function of OX4OL is not shared by two other costimulatory TNF-family members, GITR-ligand and 4-1BB-ligand. It has been shown that signaling the OX40-receptor on human T cells by antibodies, small molecules, or the OX4OL modulates the generation and function of IL-10 producing Foxp3+ Treg immunosuppressive T cells and blocks Foxp3+ Treg function. Further, provided are high throughput methods for identifying compounds that can inhibit the immunosuppressive function of IL-10 producing Tr1 cells.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
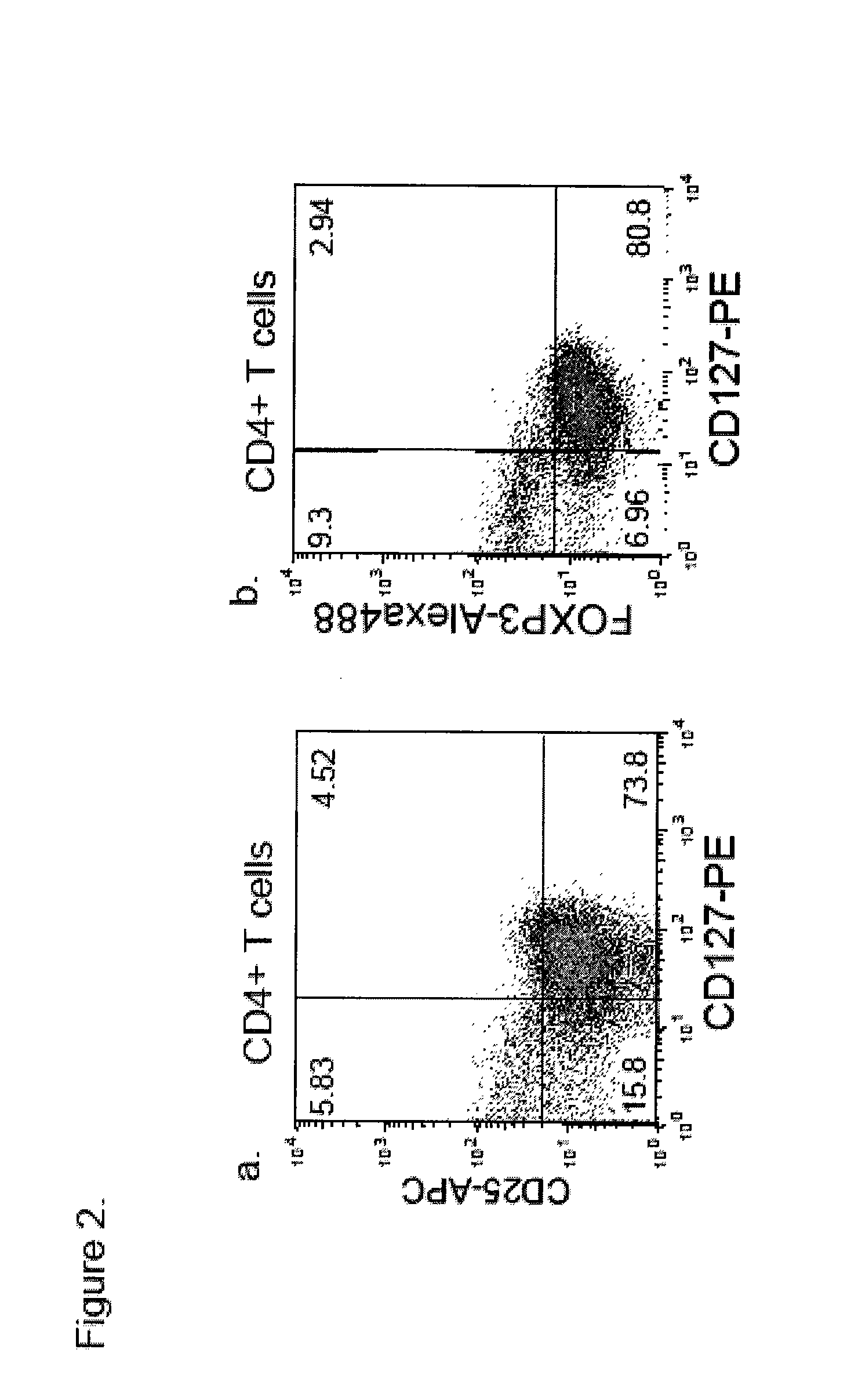
Cd127 expression inversely correlates with foxp3 and suppressive function of cd4+ tregs
The invention provides methods of isolating CD127lo / − immunosuppressive regulatory T cells which can be greatly enriched for FoxP3, methods of expanding the isolated cells, pharmaceutical compositions of such cells, and methods of their use in the treatment of autoimmune and other immune system mediated disorders.
Owner:RGT UNIV OF CALIFORNIA
Compositions and methods for modulation of suppressor t cell activation
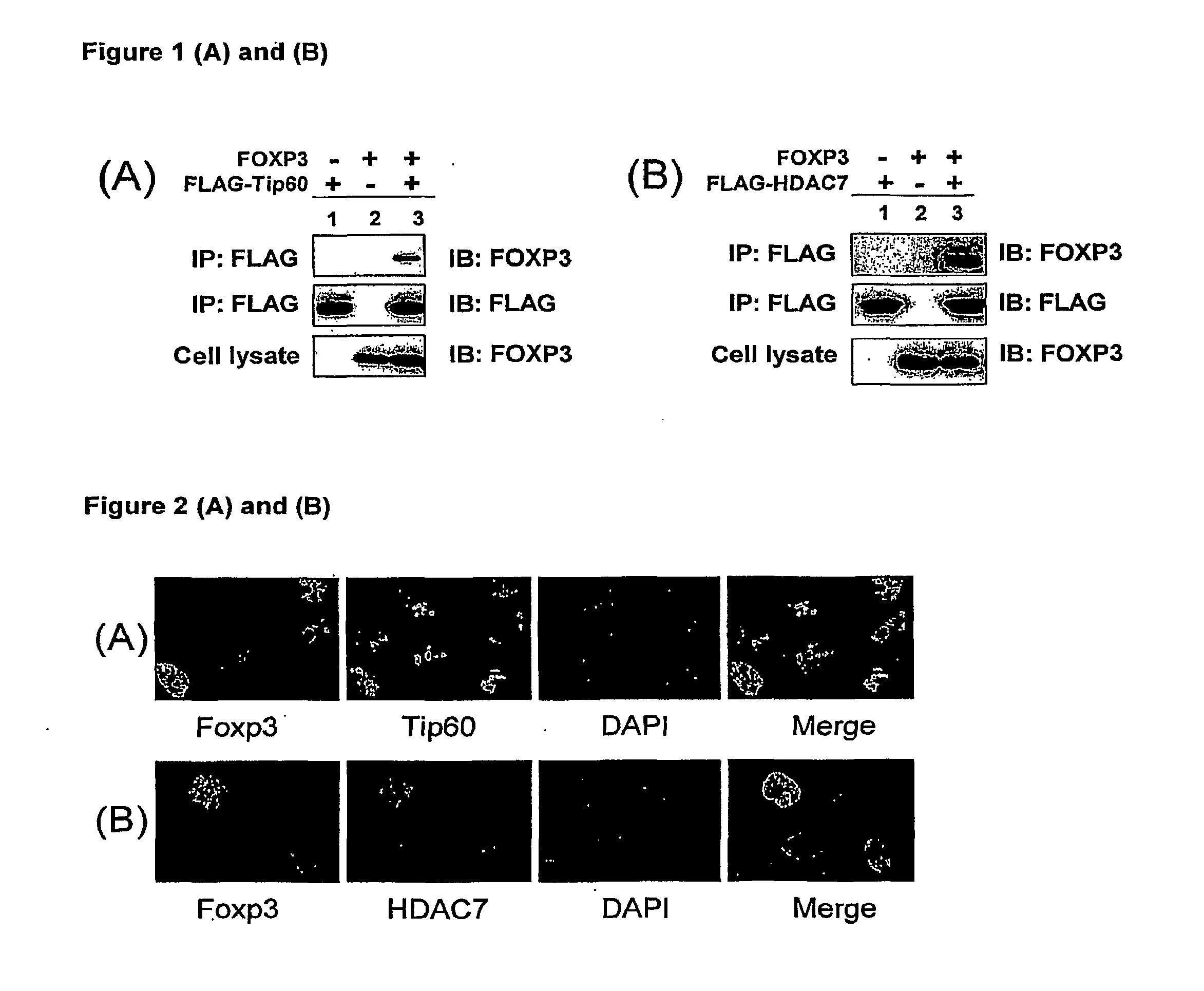
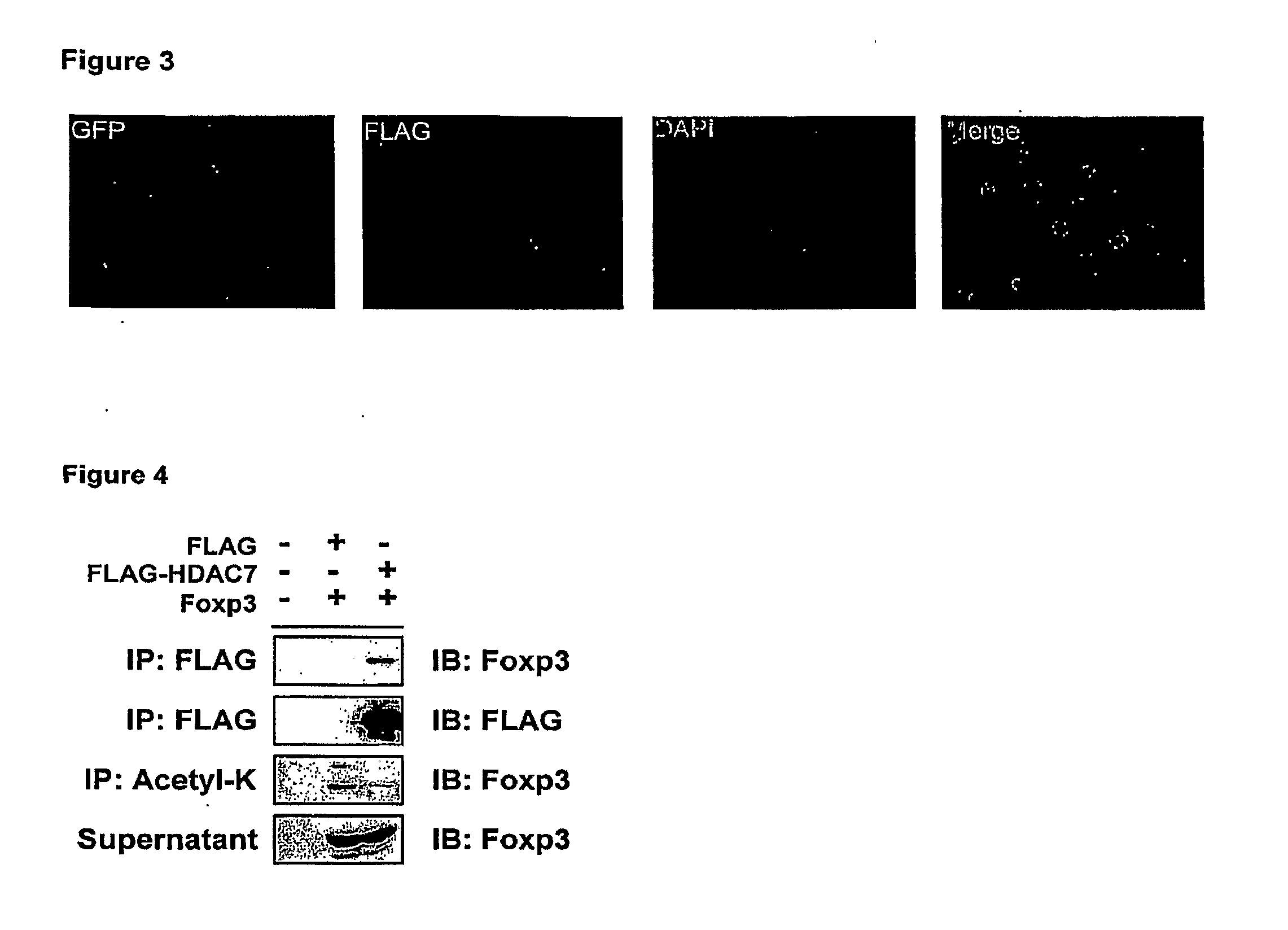
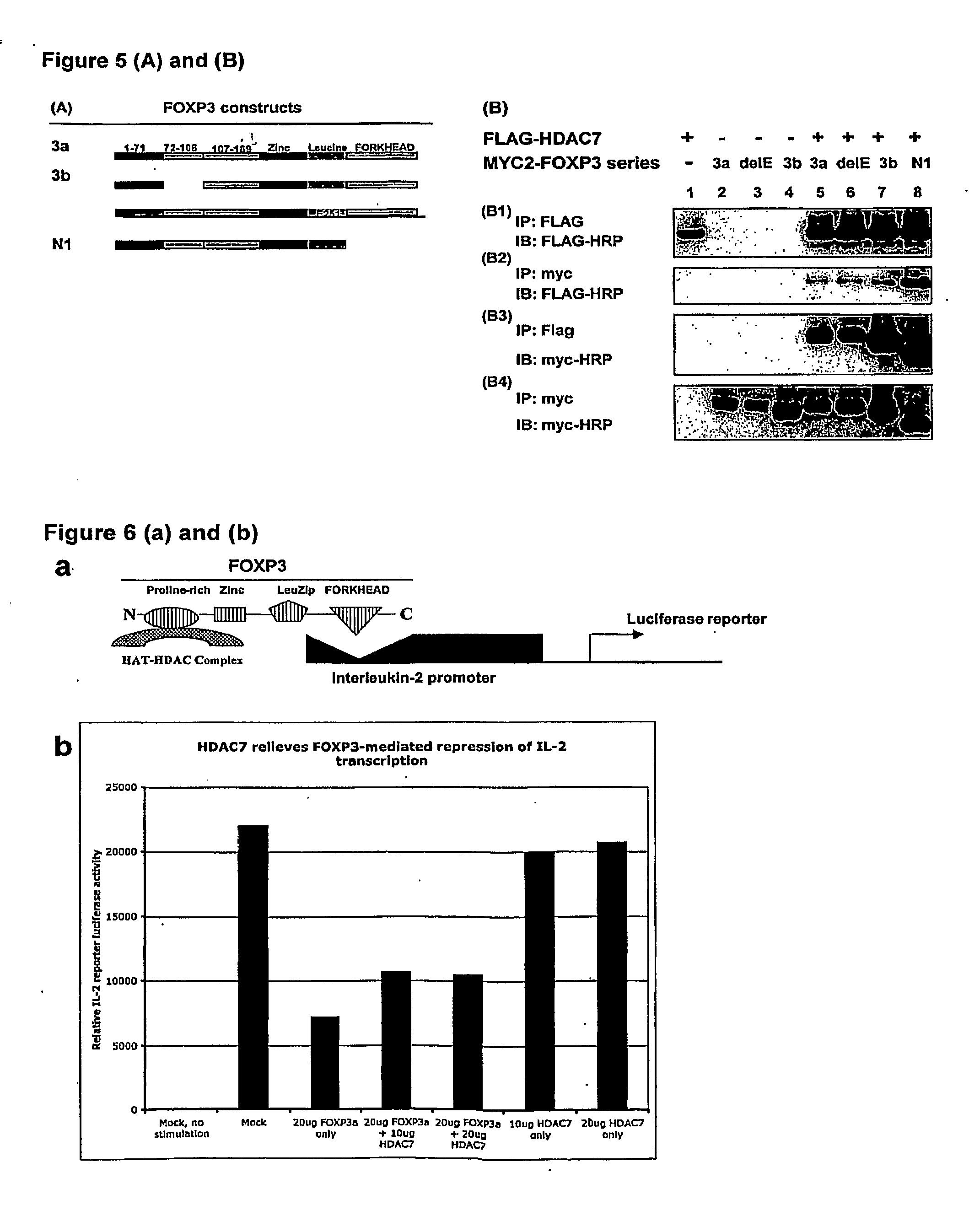
InactiveUS20100061984A1Relieve symptomsIncreased acetylation levelsCompounds screening/testingAntibacterial agentsAntigenImmunologic disorders
Methods of treating autoimmune disorders, coronary artery disease, allergy symptoms, allograft rejection sepsis / toxic shock are disclosed. Some methods comprise administering one or more regulatory compositions to activate the T suppressor cells by increasing the acetylation level and / or protein level of FOXP3 in combination with a T suppressor stimulus and / or an antigen. Some methods comprise administering one or more regulatory compositions to activate the T suppressor cells by increasing the acetylation level and / or protein level of FOXP3. Some methods comprise administering soluble GITR or antibodies that bind to GITR ligand. Methods of treating cancer, infectious diseases, and immune deficiency are also disclosed as are vaccination methods. The methods comprise administering one or more regulatory compositions to inactivate the T suppressor cells by reducing the acetylation level and / or protein level of FOXP3. Improved vaccines and vaccination methods are disclosed. Methods of identifying compounds that are useful to modulate acetylation level and / or protein level of FOXP3 and treat diseases are disclosed.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
Method for in situ inhibition of regulatory t cells
ActiveUS20170067022A1Reducing FoxP transcriptional activityHydrolasesAntibody mimetics/scaffoldsAntigenInfected cell
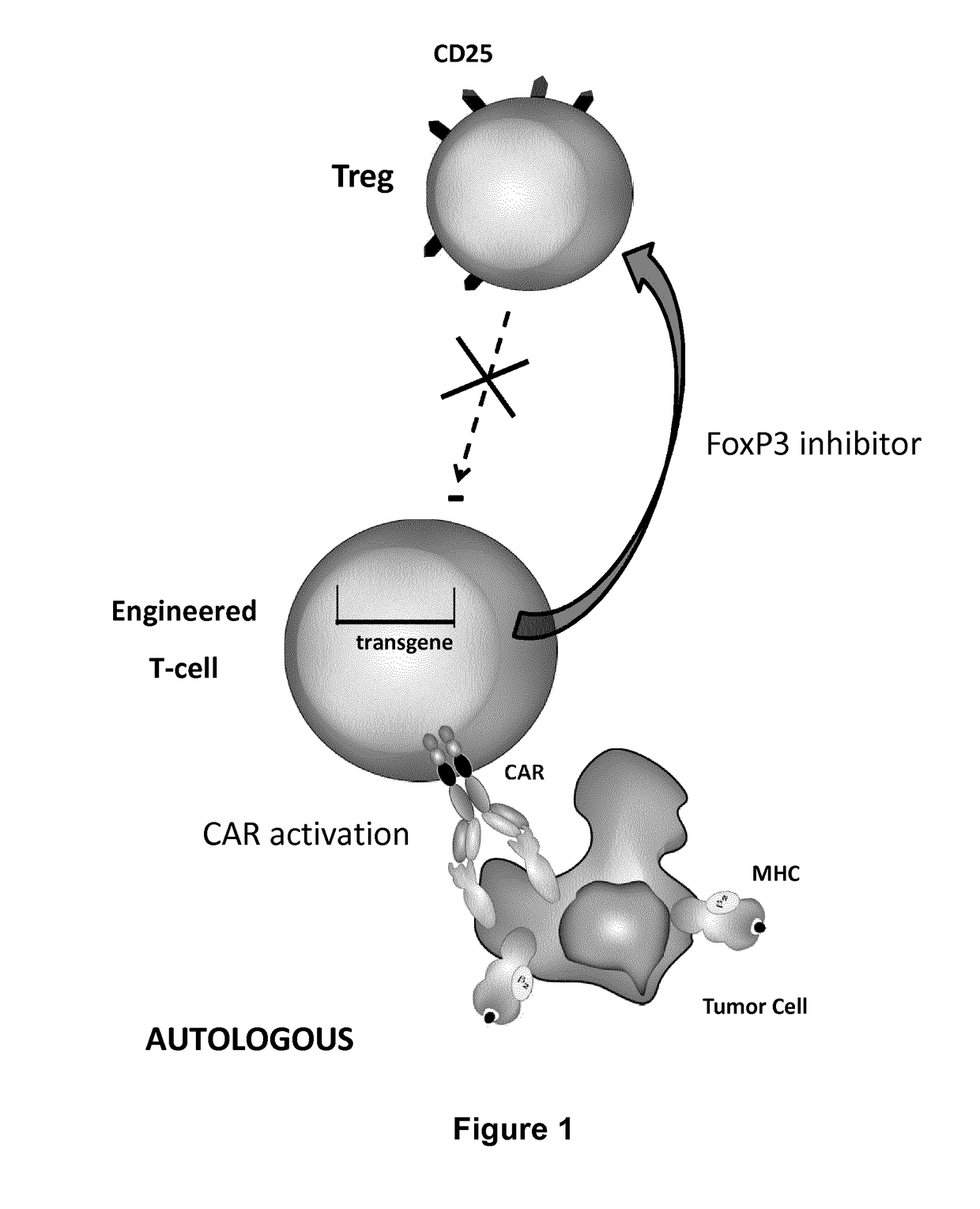
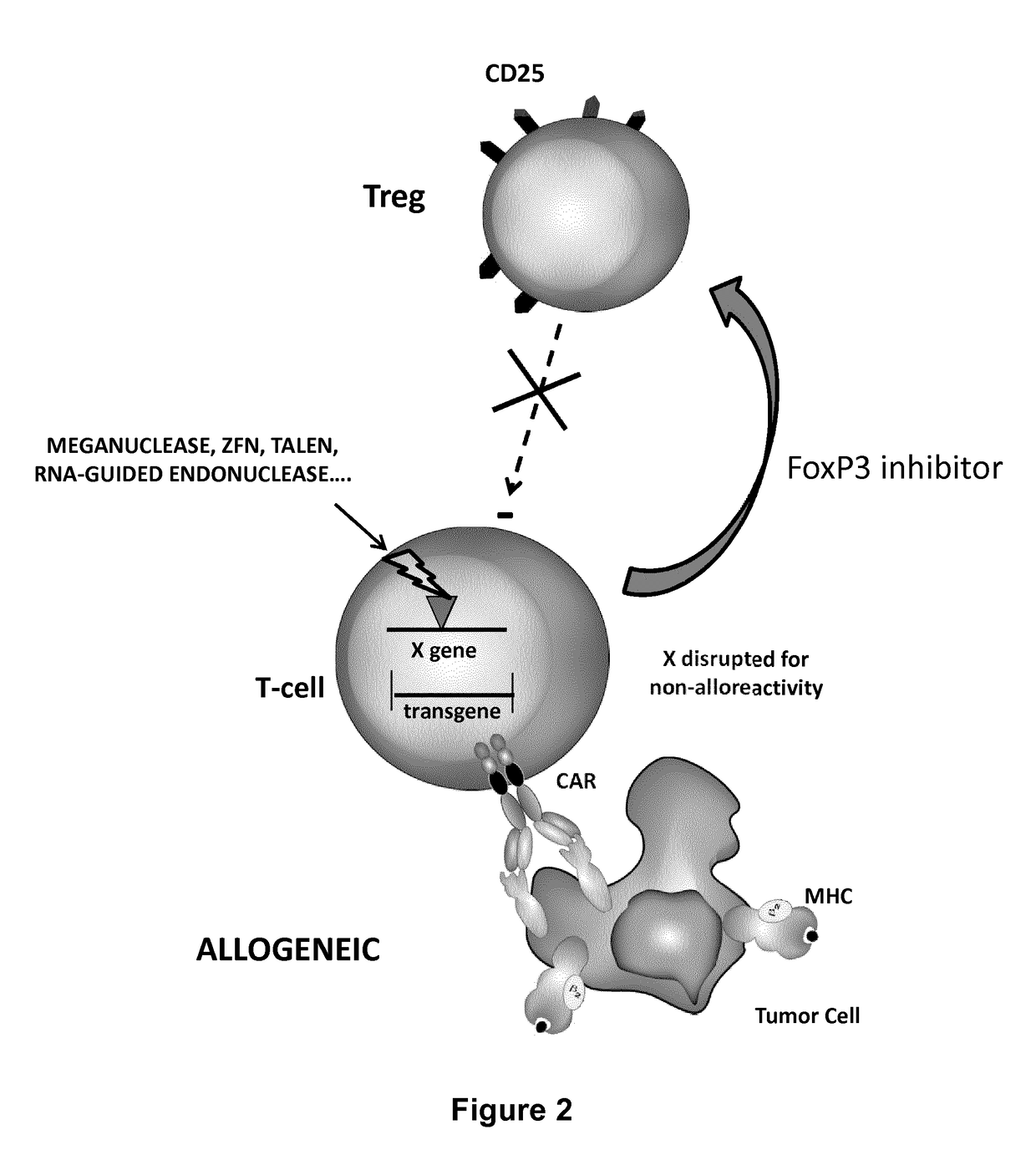
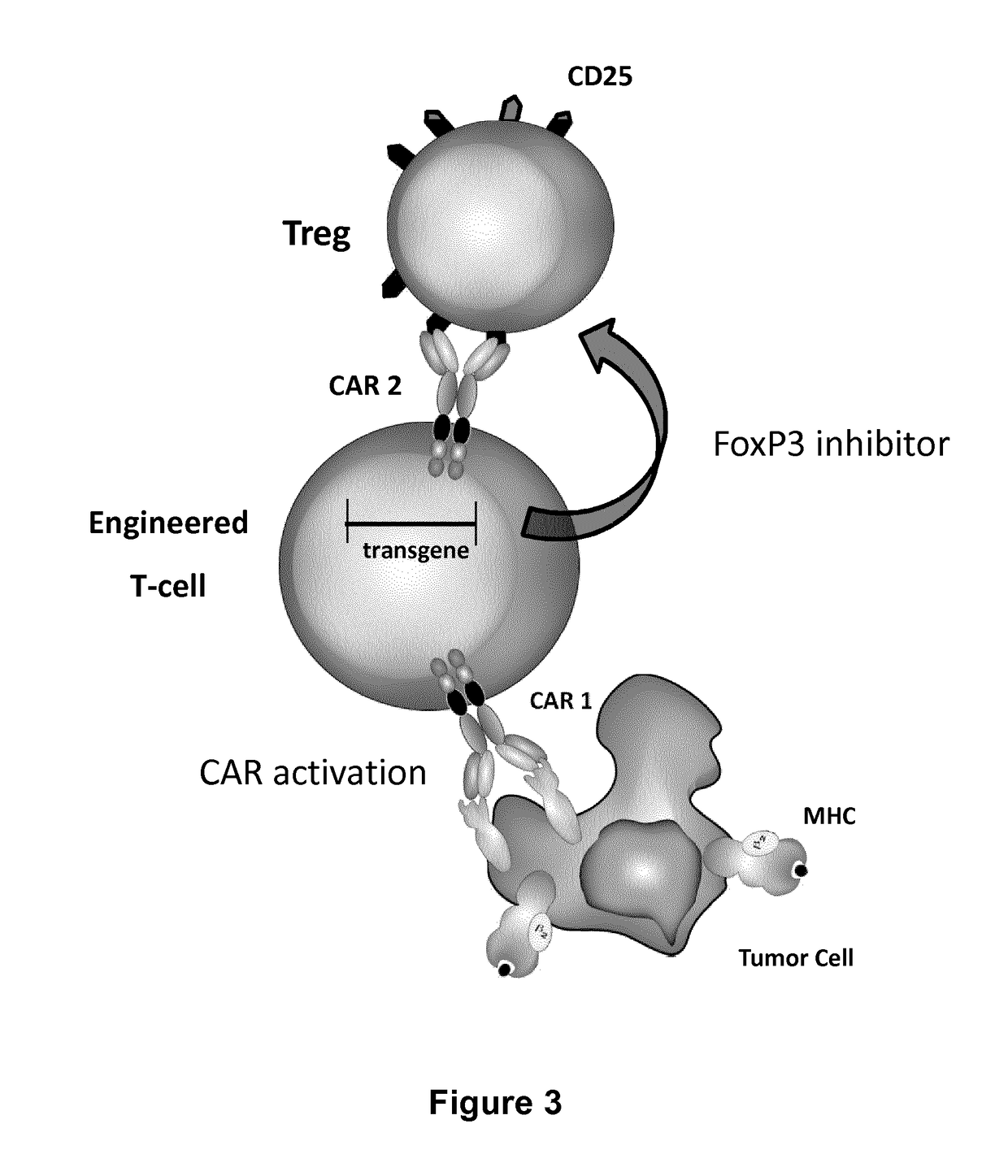
The present invention pertains to engineered T-cells, method for their preparation and their use as medicament, particularly for immunotherapy. The engineered T-cells of the invention are designed to express both a Chimeric Antigen Receptor (CAR) directed against at least one antigen expressed at the surface of a malignant or infected cell, and a secreted inhibitor of regulatory T-cells (Treg). Preferably, such secreted inhibitor is a peptide inhibitor of forkhead / winged helix transcription factor 3 (FoxP3), a specific factor involved into the differentiation of T-cells into regulatory T-cells. The engineered T-cells of the invention direct their immune activity towards specific malignant or infected cells, while at the same time will prevent neighbouring regulatory T-cells from modulating the immune response. The invention opens the way to standard and affordable adoptive immunotherapy strategies, especially for treating or preventing cancer, and bacterial or viral infections.
Owner:CELLECTIS SA
Methods and compositions to enhance vaccine efficacy by reprogramming regulatory t cells
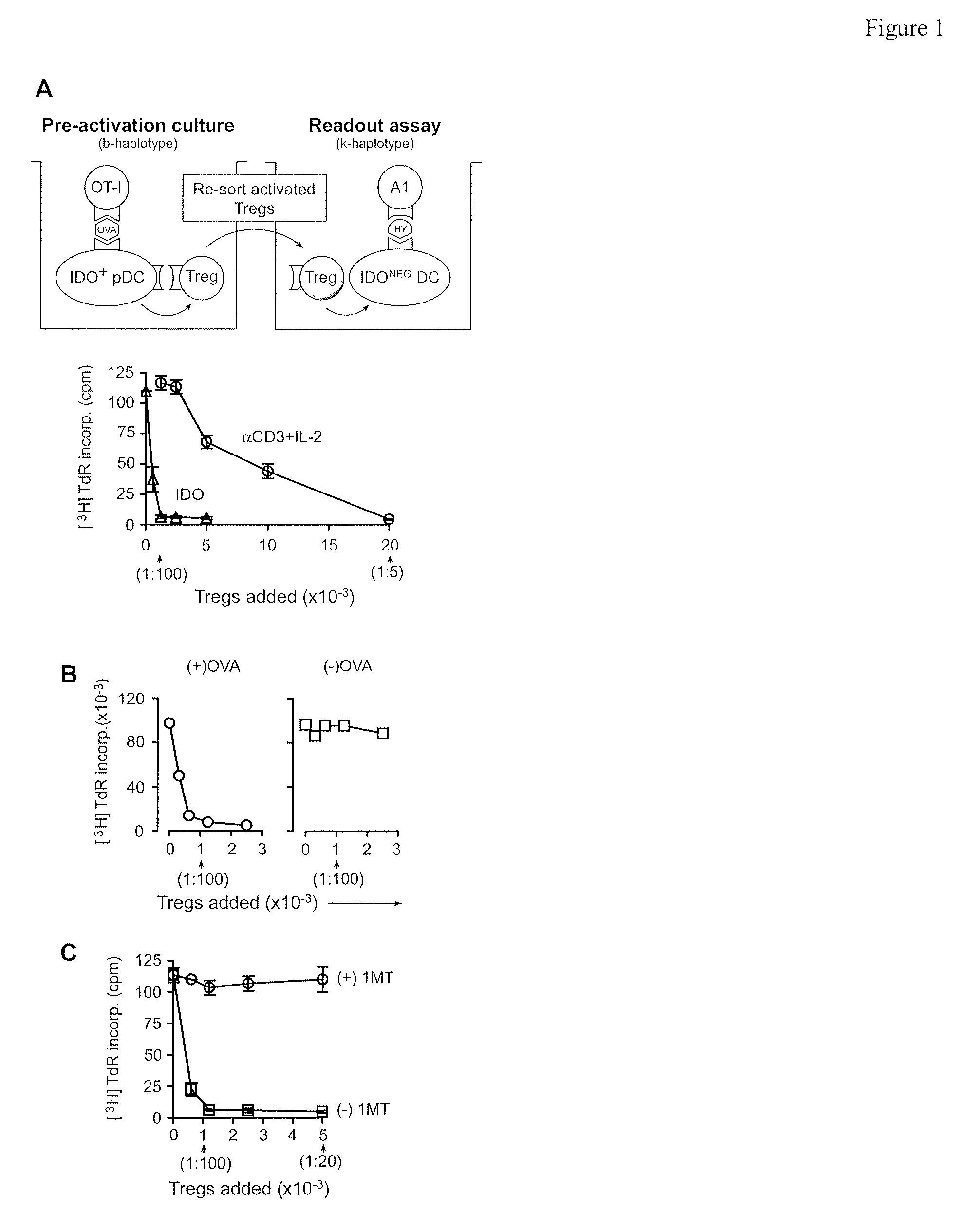
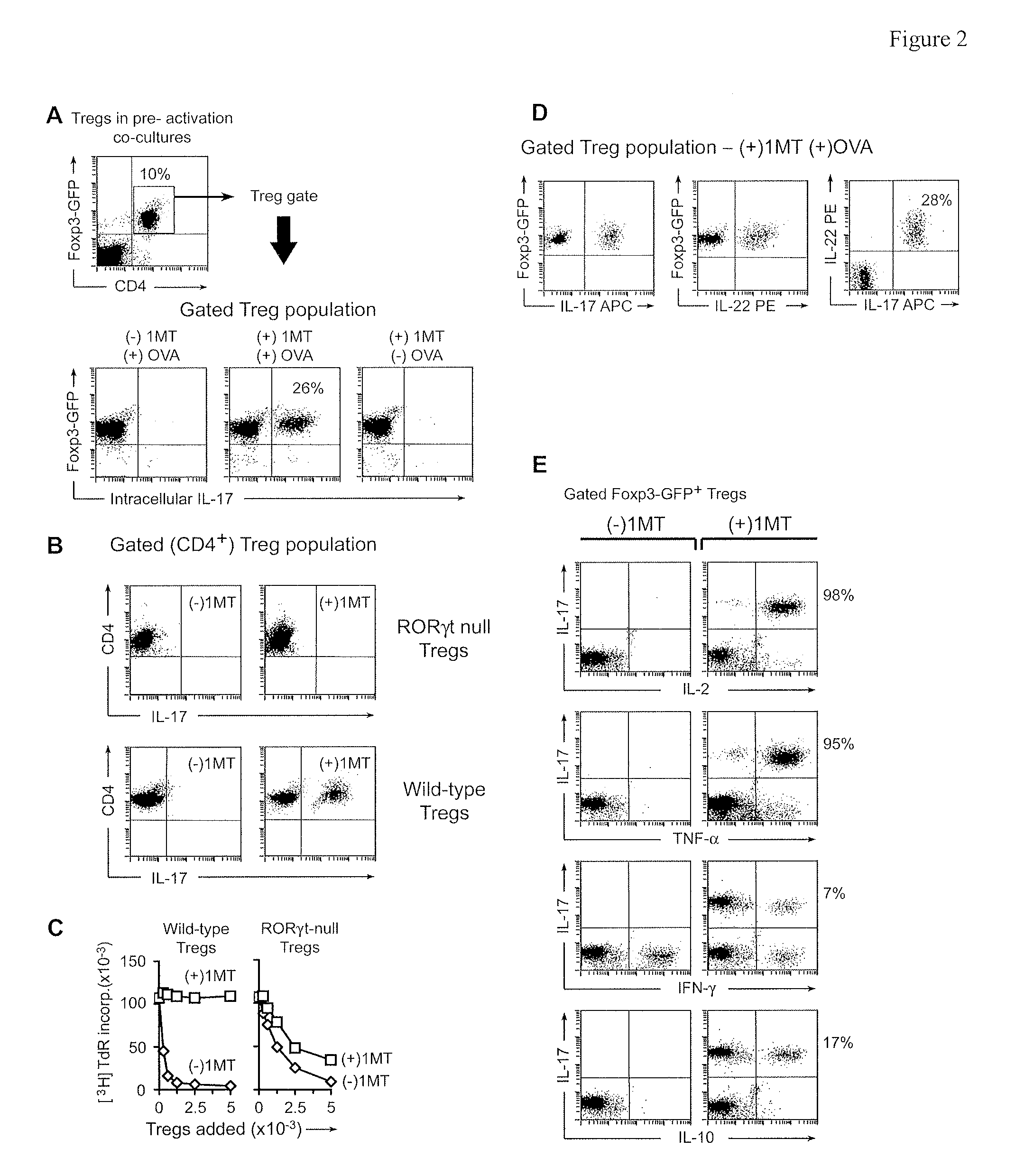
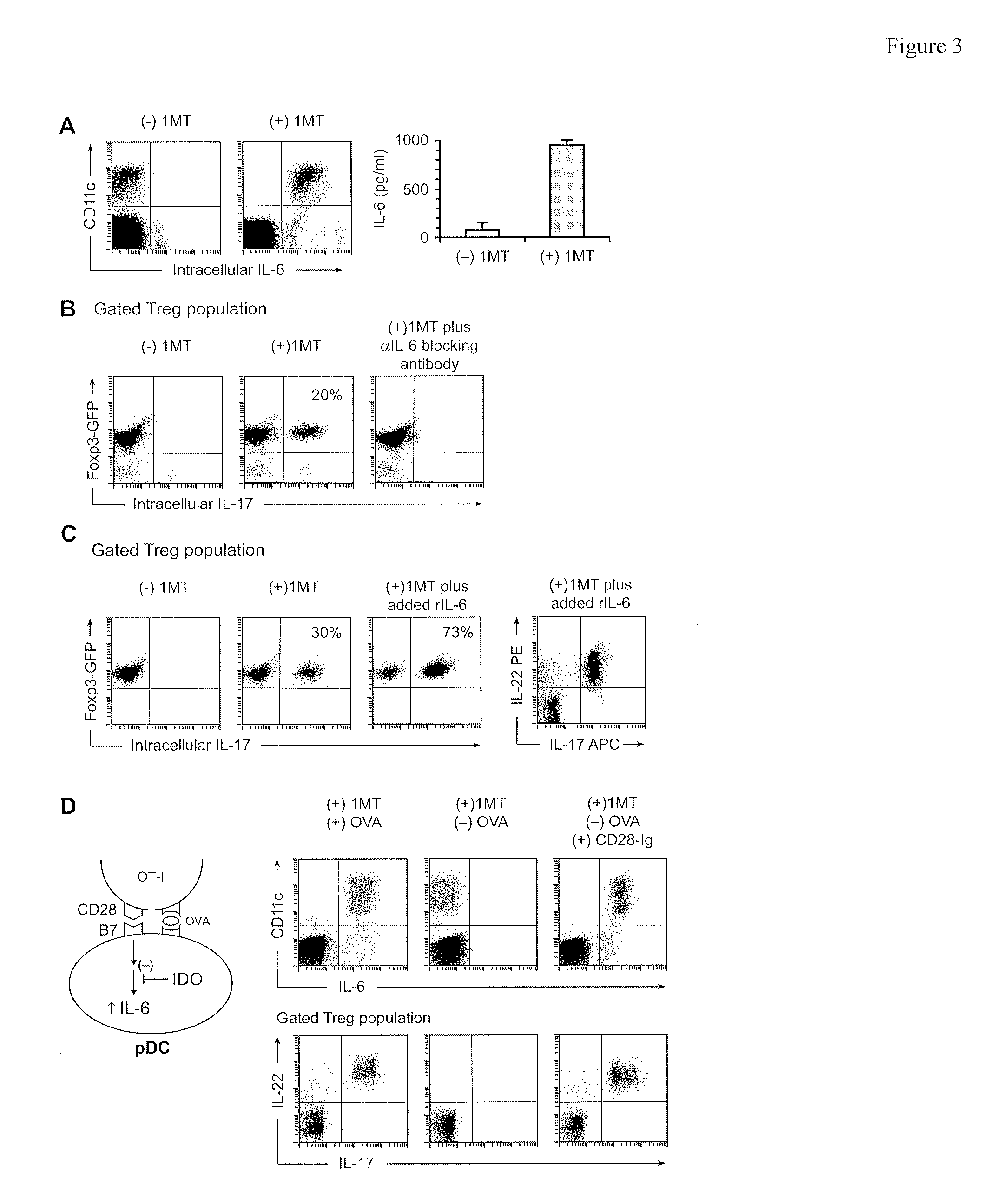
The immunoregulatory enzyme indoleamine 2,3-dioxygenase (IDO) is expressed by a subset of murine plasmacytoid DCs (pDCs) in tumor-draining LNs, where it can potently activate Foxp3 regulatory T cells (Tregs). We now show that IDO functions as a molecular switch in tumor-draining LNs, maintaining Tregs in their normal suppressive phenotype when IDO was active, but allowing inflammation-induced conversion of Tregs to a polyfunctional T-helper phenotype similar to proinflammatory TH17 cells when IDO was blocked. In vitro, conversion of Tregs to the TH17-like phenotype was driven by antigen-activated effector T cells, and required IL-6 produced by activated pDCs. IDO regulated this conversion by dominantly suppressing production of IL-6 in pDCs, in a GCN2-kinase dependent fashion. In vivo, using a model of established B16 melanoma, the combination of an IDO-inhibitor drug plus anti-tumor vaccine caused upregulation of IL-6 in pDCs and in situ conversion of a majority of Tregs to the TH17 phenotype, with marked enhancement of CD8+ T cell activation and anti-tumor efficacy. Thus, Tregs in tumor-draining LNs can be actively re-programmed in vitro and in vivo into T-helper cells, without the need for physical depletion, and IDO serves as a key regulator of this critical conversion.
Owner:GEORGIA HEALTH SCI UNIV RES INST
Methods for generating antigen-specific effector T cells
InactiveCN101415827AImmunoglobulin superfamilyMicroinjection basedAutoimmune conditionAutoimmune disease
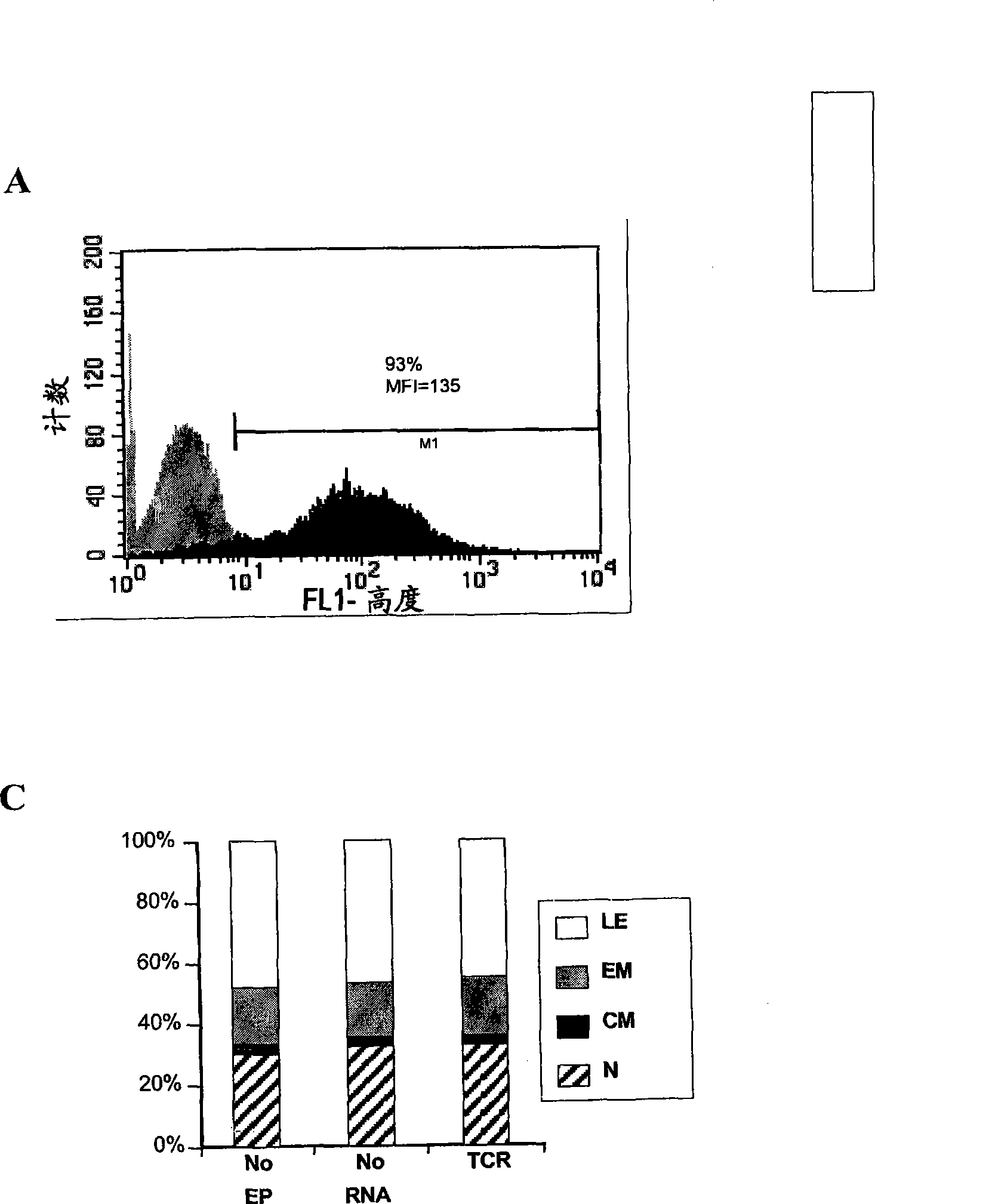
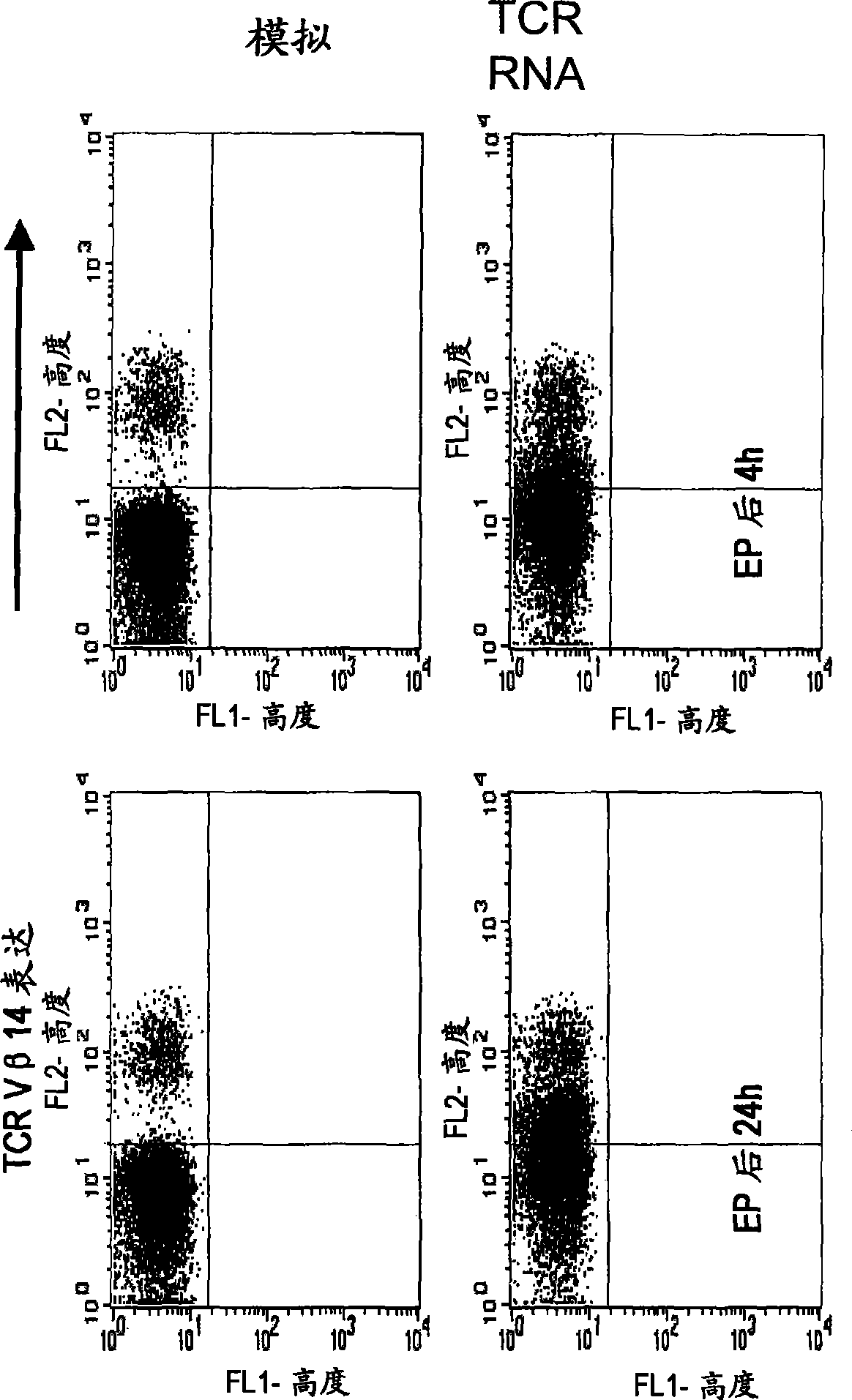
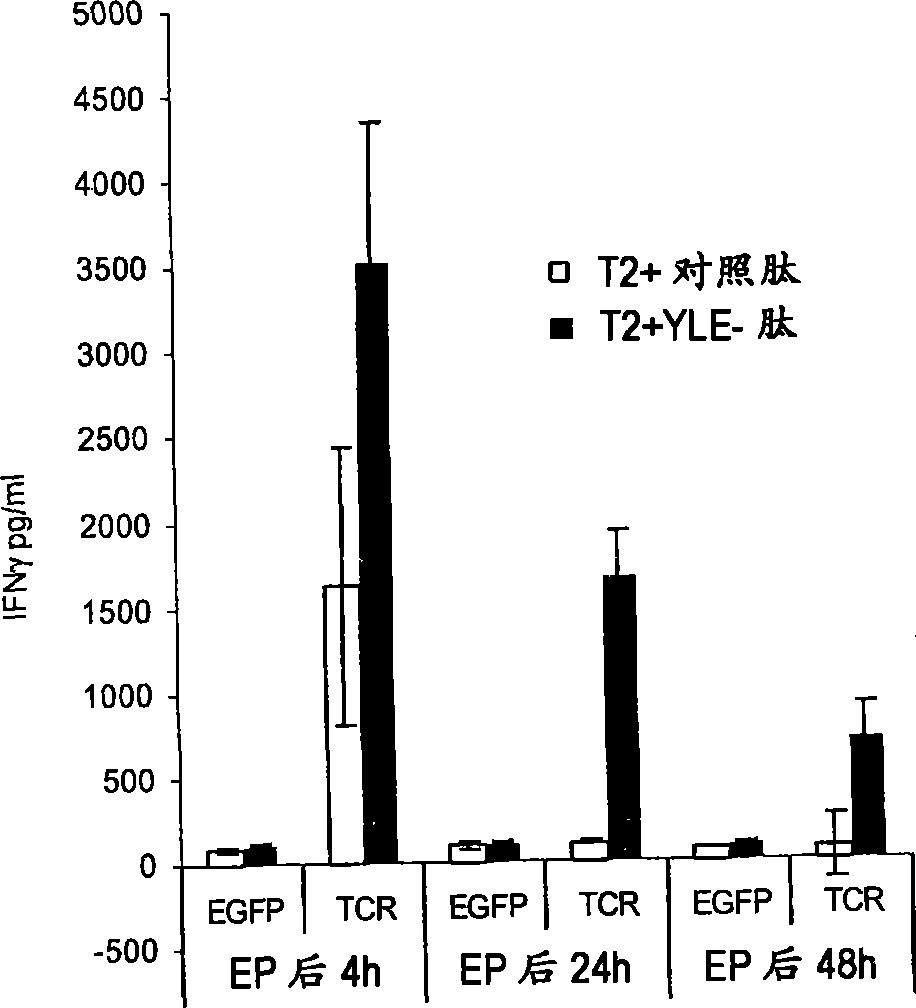
The invention relates to T cells transiently transfected with RNA, especially RNA encoding a T cell receptor and / or FoxP3, and methods of transfecting T cells with RNA by electroporation. Compositions of the invention include an effector T cell transiently transfected with RNA encoding a T cell receptor (TCR) specific for an antigen, wherein the T cell demonstrates effector function specific for cells presenting the antigen in complex with an MHC molecule. Treg cells comprising an exogenous RNA encoding FoxP3 are also provided. The transfected T cells are useful for immunotherapy, particularly in the treatment of tumors, pathogen infection, autoimmune disease, transplant rejection and graft versus host disease.
Owner:ARGOS THERAPEUTICS INC
Materials And Methods For FOXP3 Tumor Suppression
Provided herein are methods of treating a cancer in a subject comprising administering a FOXP3 protein, a nucleic acid encoding a FOXP3 protein, or an inducing compound which induces FOXP3 protein expression. Methods of altering a phenotype of a cancer cell or tumor cell, methods of inhibiting growth of such cells, and methods of inducing apoptosis of these cells are also provided herein. These methods comprise contacting the cell with a FOXP3 protein, a nucleic acid encoding a FOXP3 protein, or an inducing compound which induces FOXP3 protein expression. Further provided herein are diagnostic methods, comprising comparing the expression or structure of a FOXP3 protein or FOXP3 gene in a test sample to that of a normal or prior sample. A method of screening a test compound for anti-cancer activity comprising administering to cells the test compound and measuring FOXP3 protein or FOXP3 gene expression is moreover provided herein.
Owner:THE OHIO STATE UNIV RES FOUND +1
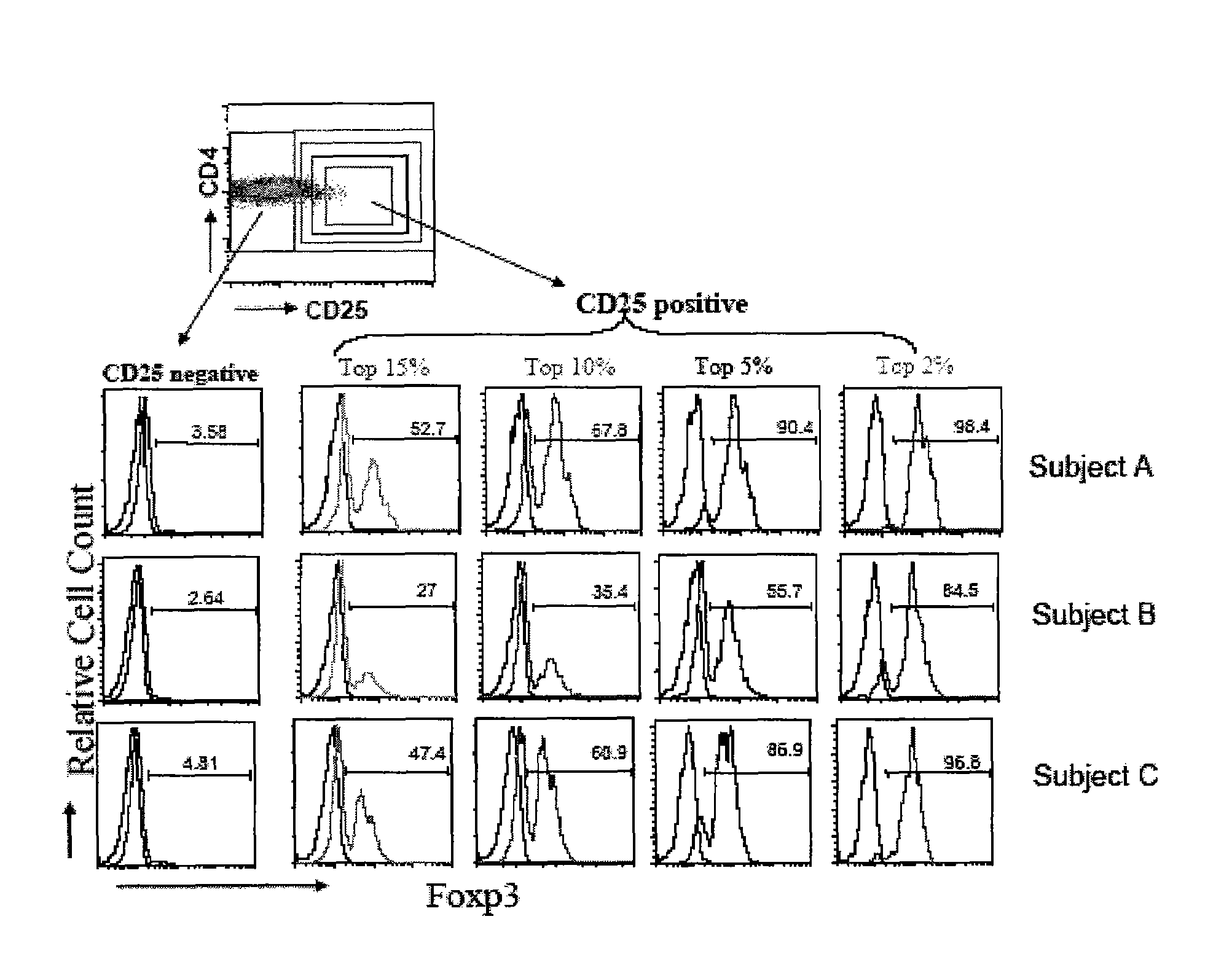
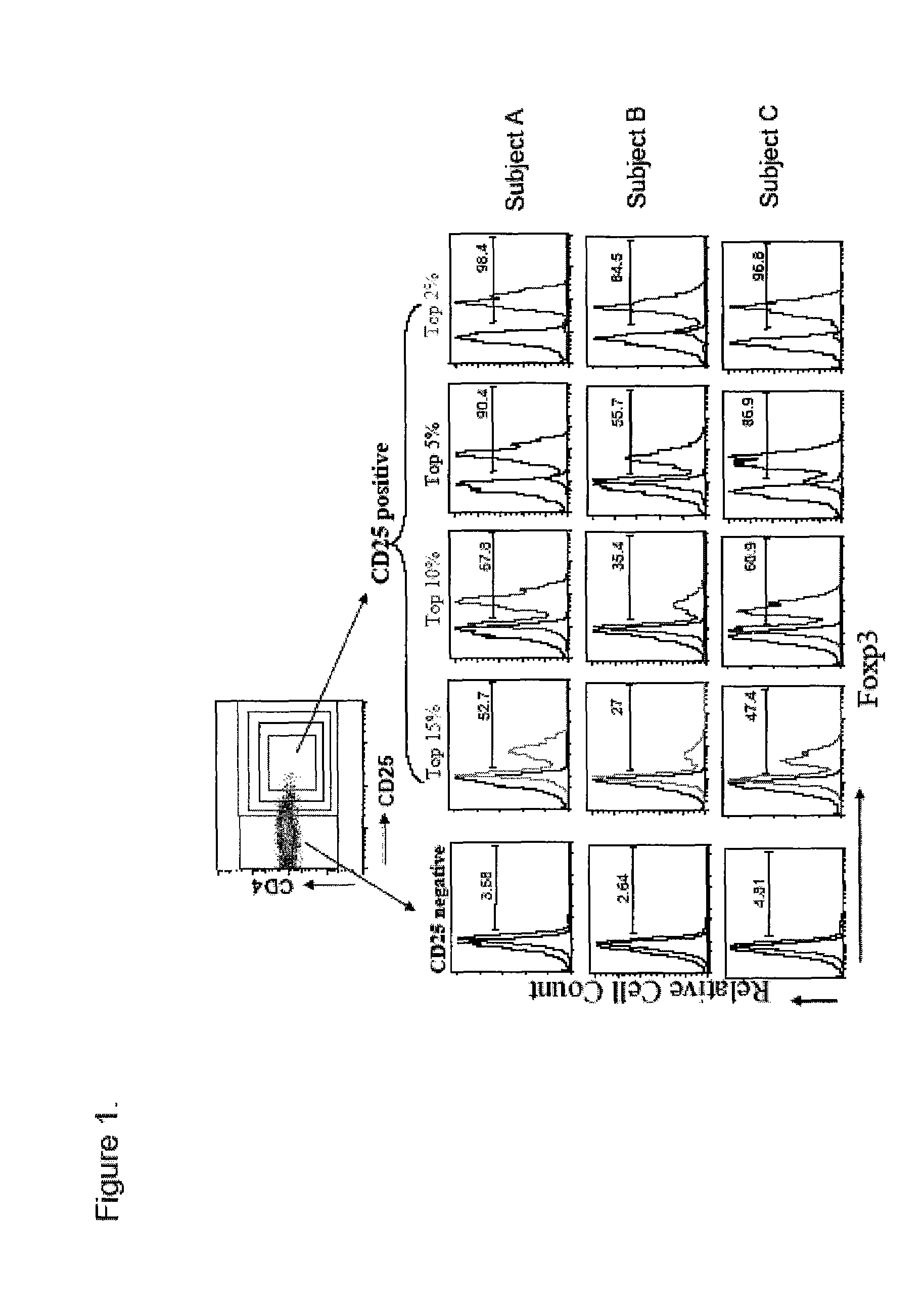
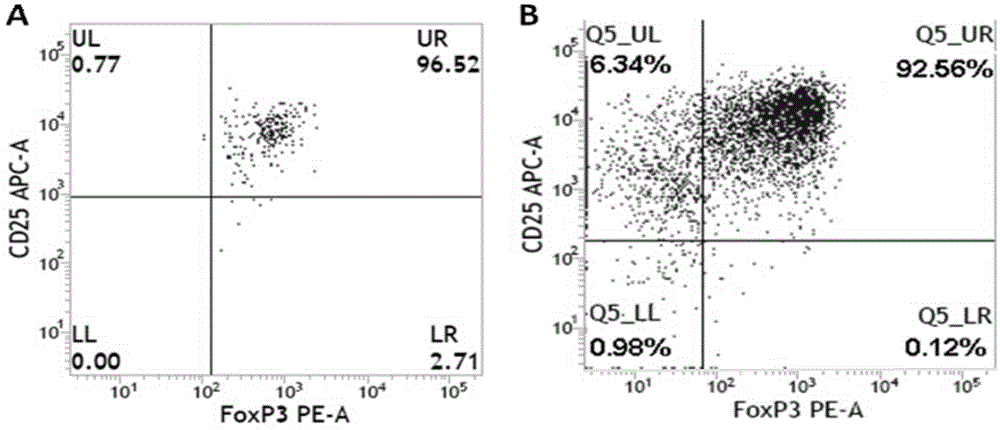
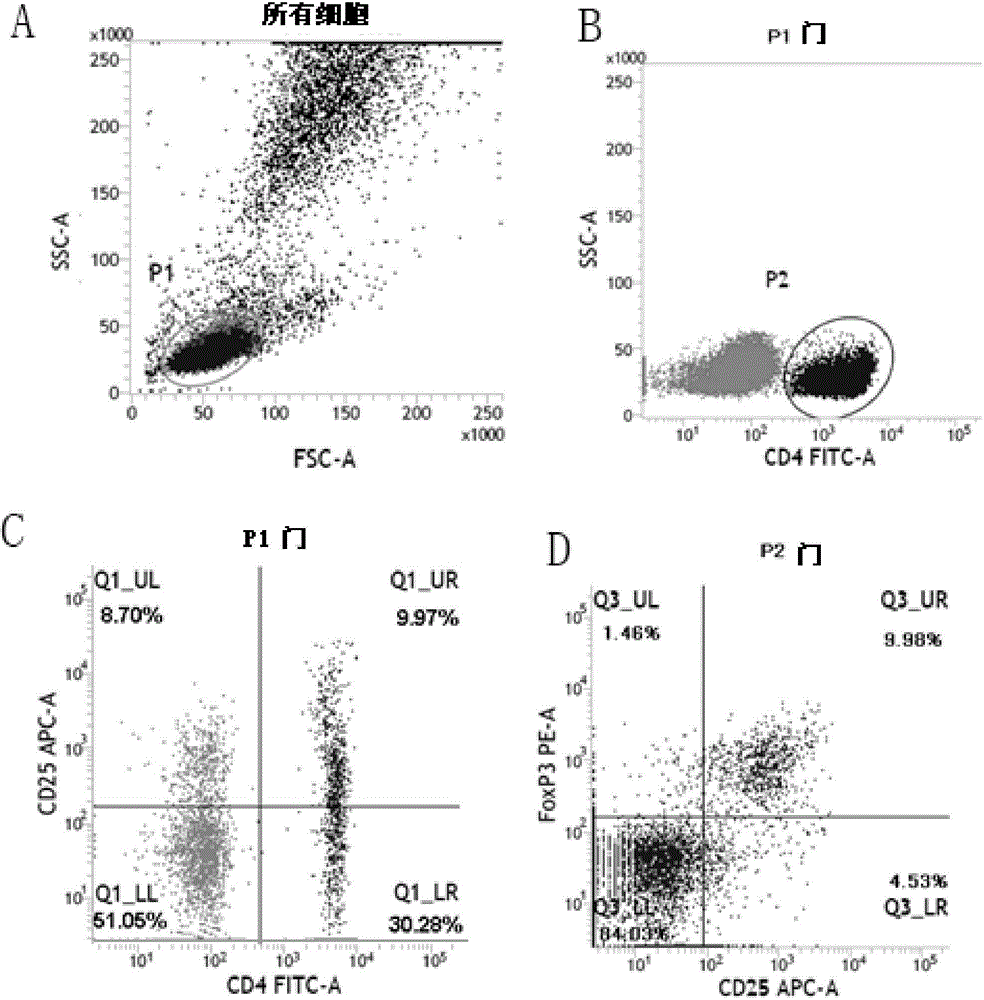
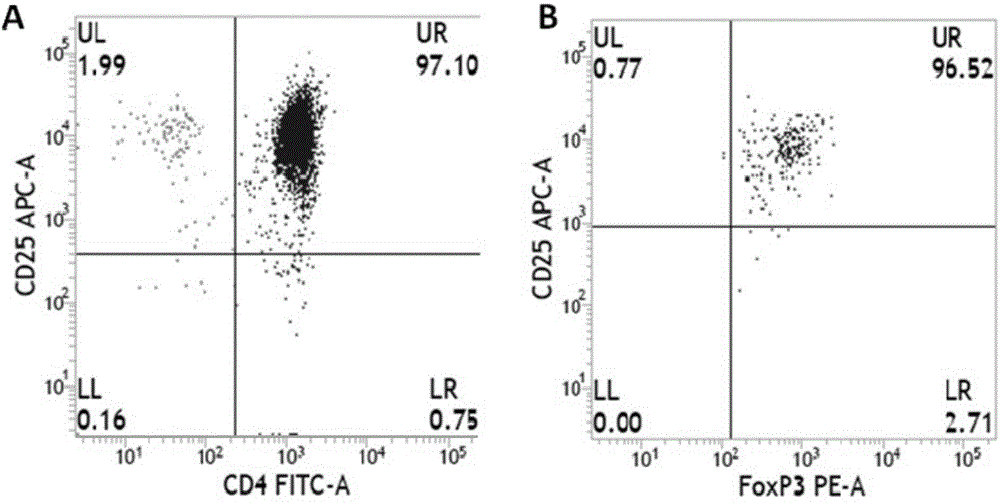
Sorting and amplification method of human peripheral blood CD4+CD25+Foxp3+ regulatory T cells
The invention discloses a sorting and amplification method of human peripheral blood CD4+CD25+Foxp3+ regulatory T cells, and belongs to the field of cell sorting amplification. The sorting and amplification method of the human peripheral blood CD4+CD25+Foxp3+ regulatory T cells specifically comprises the following steps: (1) separating peripheral blood mononuclear cells; (2) performing MACS (magnetic activated cell sorting) on CD4+CD25+Foxp3+Treg cells; and (3) performing in-vitro culture and amplification on the CD4+CD25+Foxp3+Treg cells, and finally performing amplification and culture to obtain the human peripheral blood CD4+CD25+Foxp3+ regulatory T cells. According to the sorting and amplification method disclosed by the invention, human peripheral blood CD4+CD25+Treg cells are successfully separated by using an immuno-magnetic bead two-step process, and the purity of the human peripheral blood CD4+CD25+Treg cells is subjected to flow identification to ensure that the purity of the finally-obtained CD4+CD25+Treg cells can reach 97%, and the activity of the cells can reach more than 95%.
Owner:SUN YAT SEN MEMORIAL HOSPITAL SUN YAT SEN UNIV
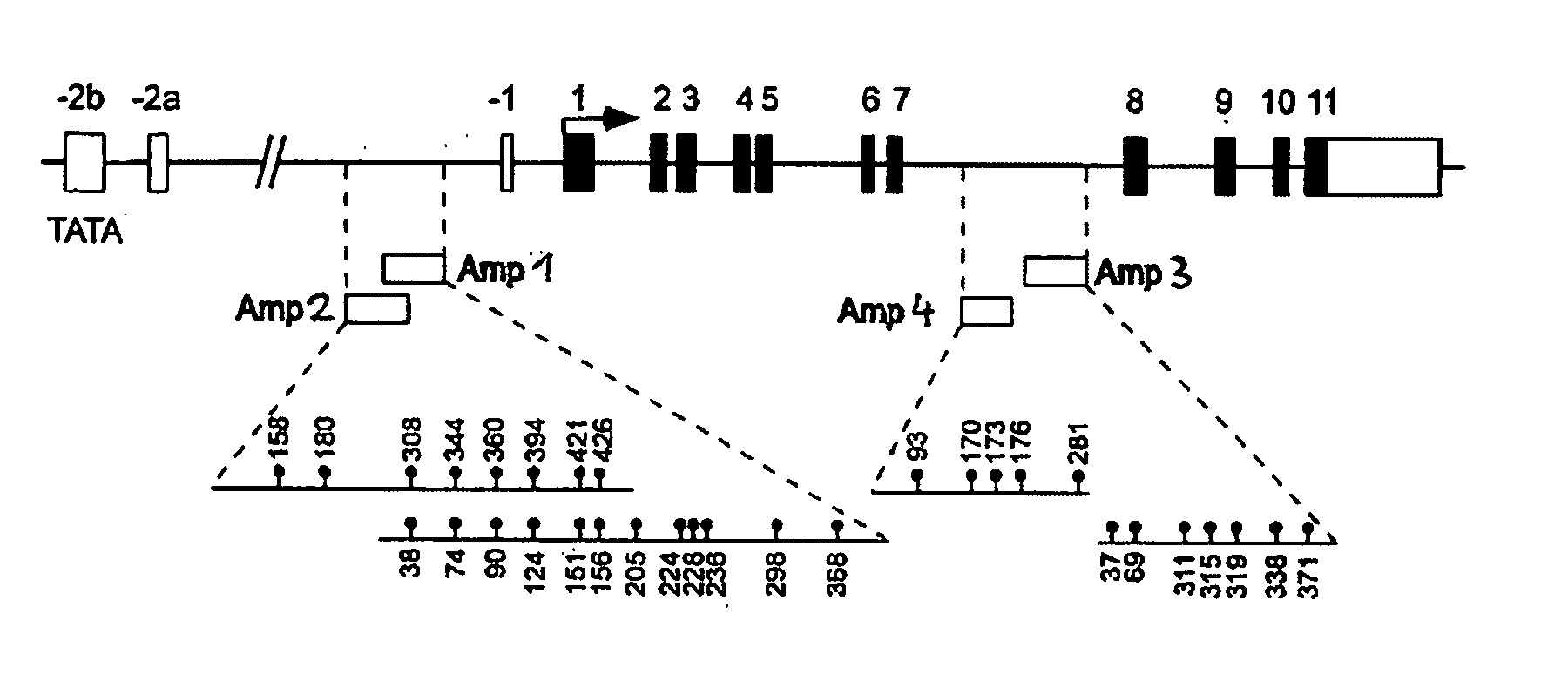
Detection and quality control of regulatory T cells through DNA-methylation analysis of the Foxp3 gene
ActiveUS20070269823A1Sugar derivativesMicrobiological testing/measurementDNA methylationRegulatory T cell
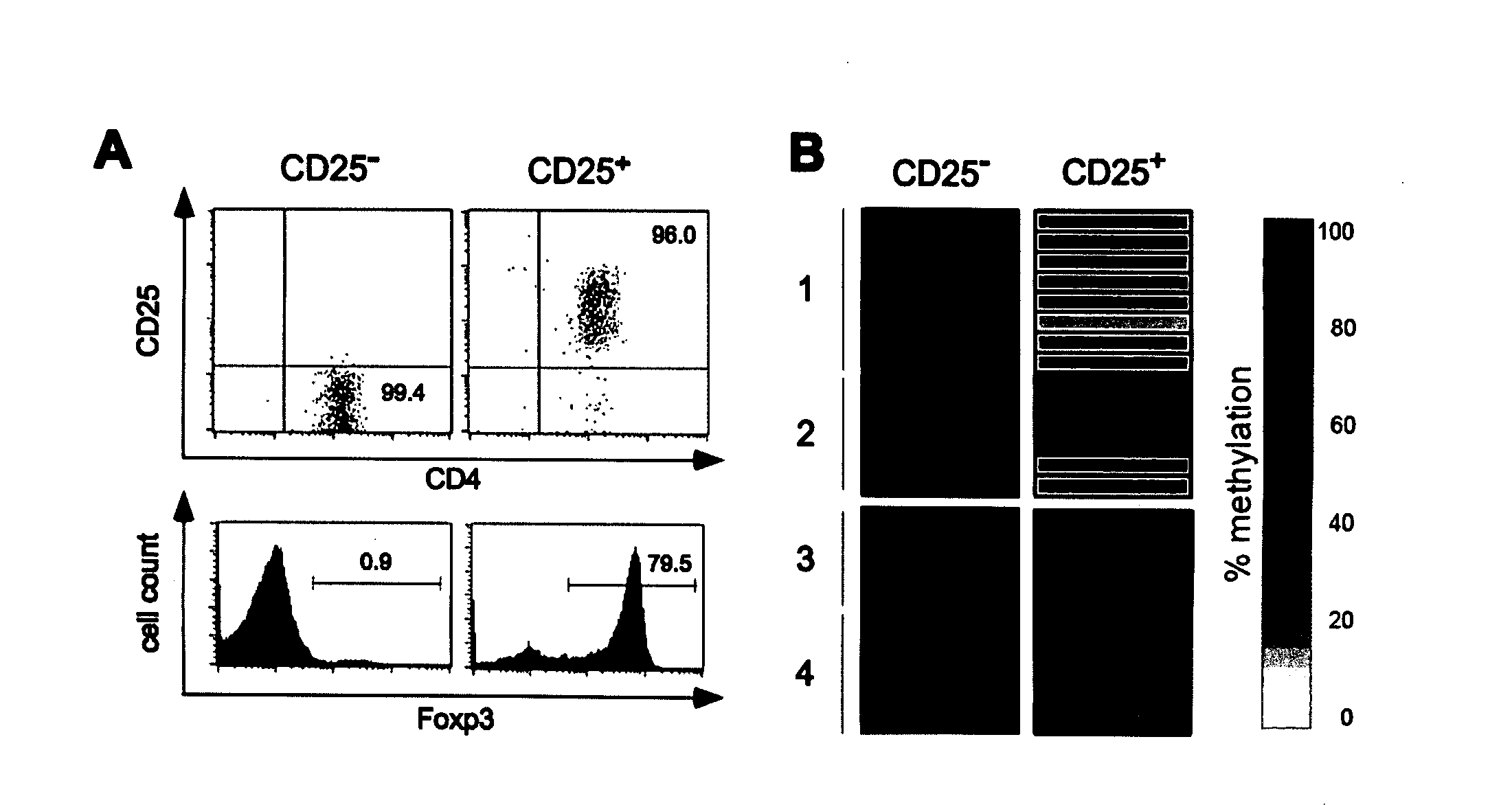
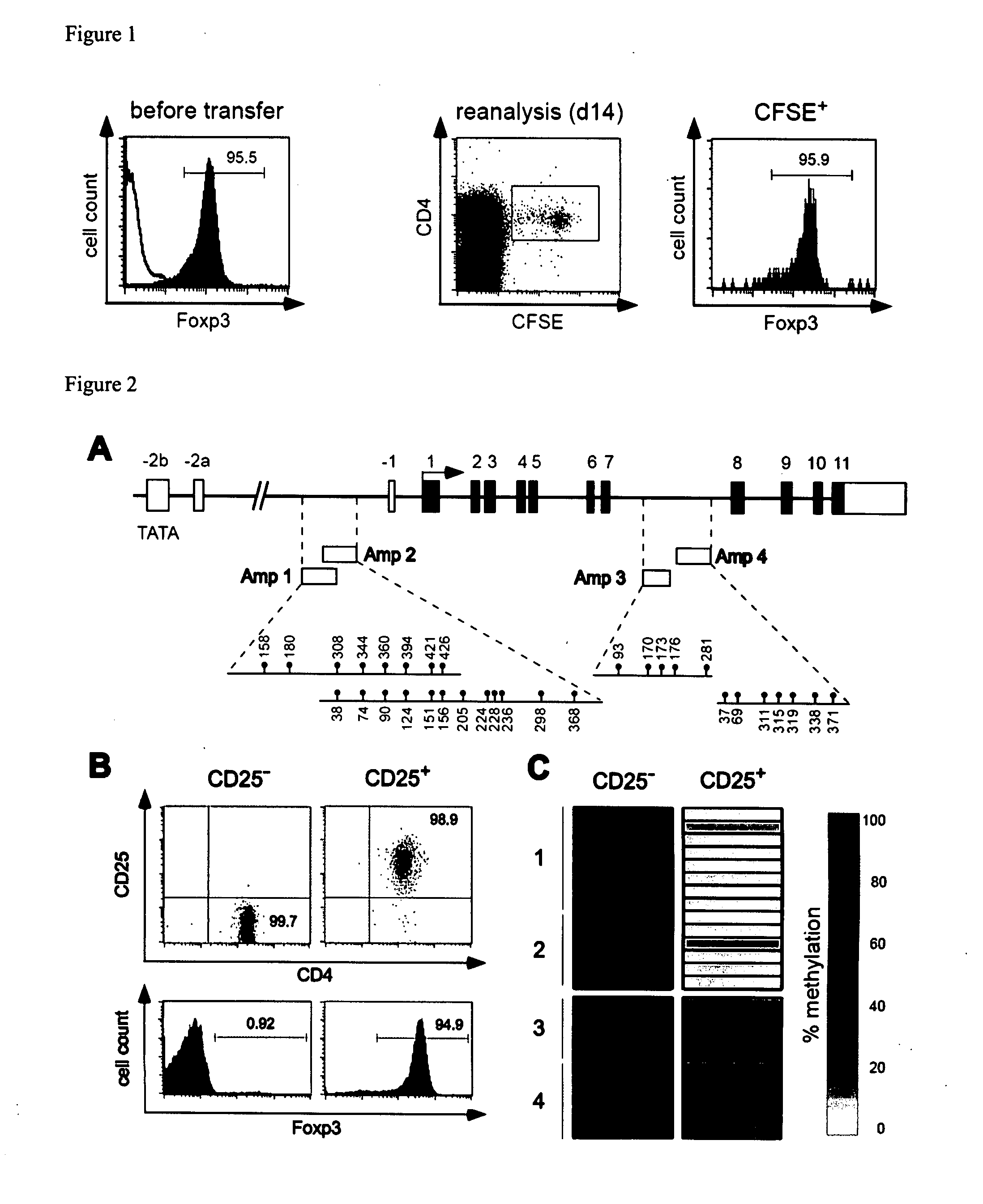
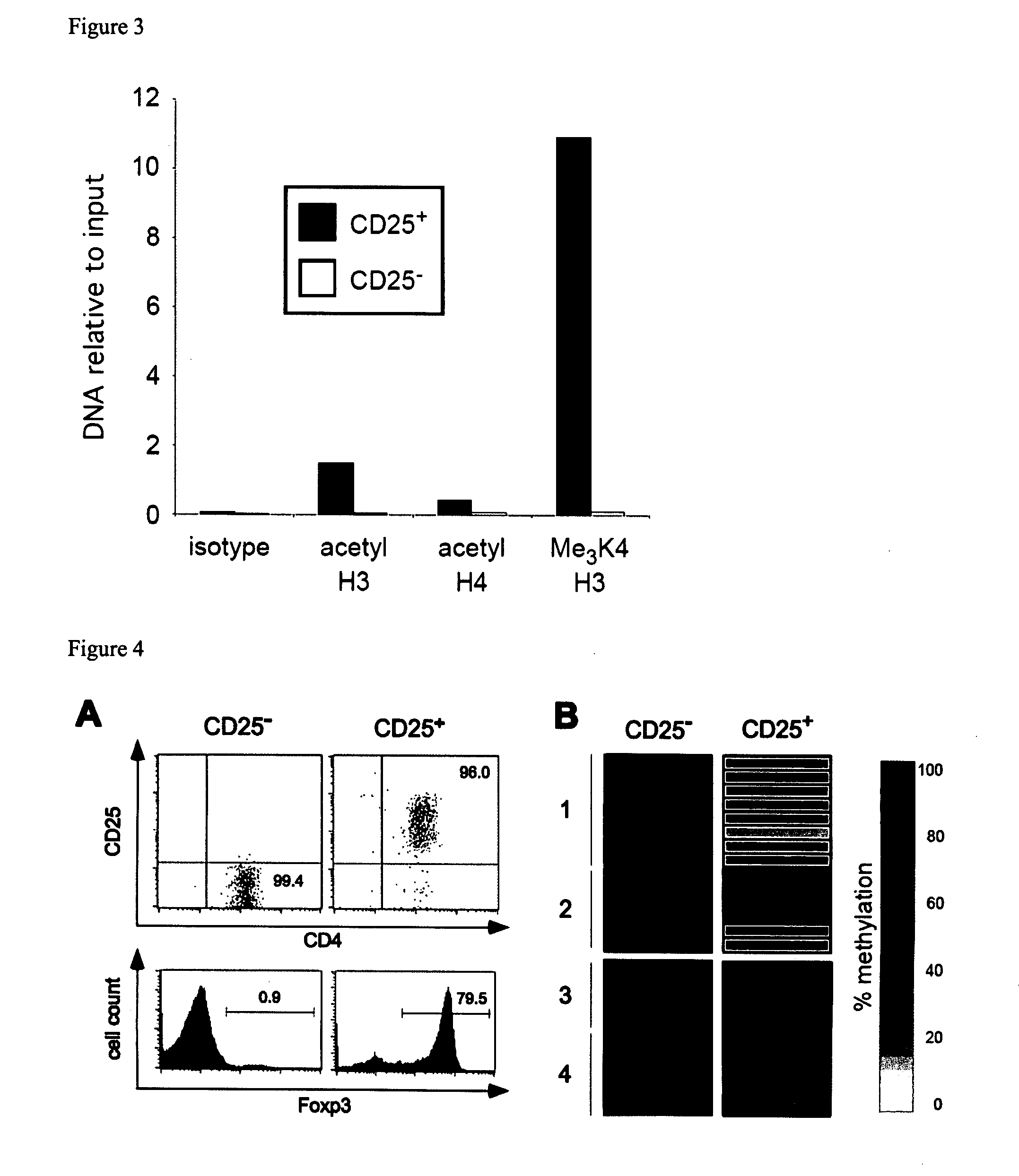
The present invention relates to a method, in particular an in vitro method for identifying FoxP3-positive regulatory T cells, preferably CD25+CD4+ regulatory T cells of a mammal, comprising analysing the methylation status of at least one CpG position in the gene foxp3 or an orthologous or paralogous gene thereof, and the use of DNA-methylation analysis of the gene of the transcription factor FoxP3 for a detection and quality assurance and control of regulatory T cells. Furthermore, the present invention relates to a kit for performing the above methods as well as respective uses.
Owner:PRECISION FOR MEDICINE GMBH +1
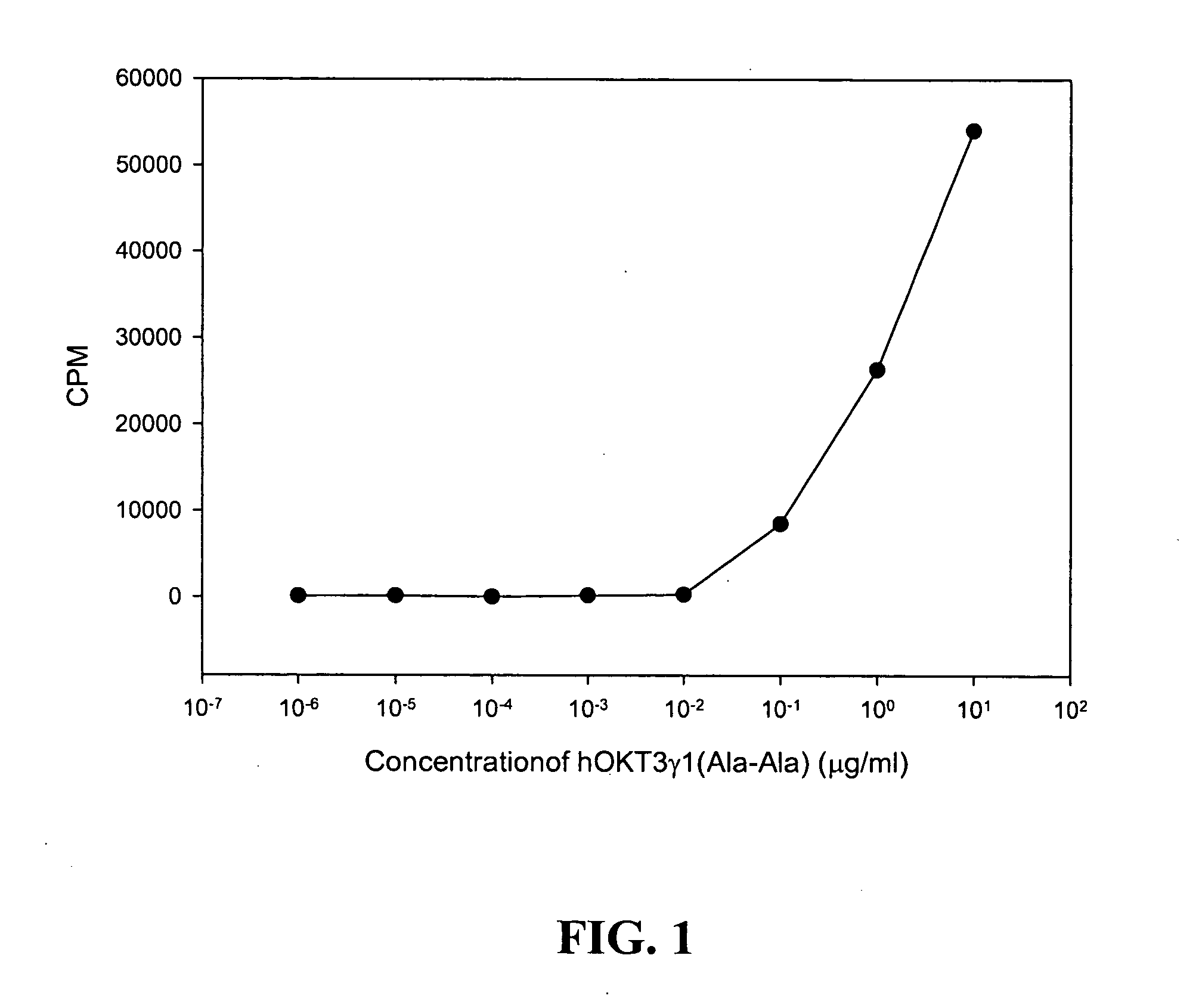
Regulatory CD8cells induced with anti-CD3 antibody
InactiveUS20070190052A1Prevent rejectionAntibody ingredientsUnknown materialsRegulatory T cellTolerability
The invention provides methods for treating autoimmunity, for reestablishing tolerance, and for generally dampening or suppressing the activation state of the immune system. The methods involve the induction or activation of a particular regulatory T cell population, characterized by its expression of CD8, CD25 and Foxp3.
Owner:RGT UNIV OF CALIFORNIA +1
Methods and compositions for accelerating the generation of regulatory t cells ex vivo
The present invention is directed to generating regulatory T cells by treating a cell culture that includes non-regulatory T cells with a regulatory composition. The invention encompasses methods utilizing a regulatory composition that includes agents that prevent methylation of the locus for the FOXP3 transcription factor, agents that accelerate differentiation of T cells into suppressor cells, and agents that are histone deacetylase inhibitors. The invention also encompasses compositions of regulatory T cells generated by culturing non-regulatory T cells with a regulatory composition as well as the use of such regulatory T cells in the treatment of autoimmune diseases and aberrant immune responses.
Owner:UNIV OF SOUTHERN CALIFORNIA
Methods of modulating the ox40 receptor to treat cancer
Numerous disease states, such as human allergic, autoimmune, and autoimmune diseases, and cancer, may be treated by targeting OX40 / OX40L. OX40L inhibits the generation of Tr1 cells from naïve and memory CD4+ T cells. This unique function of OX40L is not shared by two other costimulatory TNF-family members, GITR-ligand and 4-1BB-ligand. It has been shown that signaling the OX40-receptor on human T cells by antibodies, small molecules, or the OX40L modulates the generation and function of IL-10 producing Foxp3+ Treg immunosuppressive T cells and blocks Foxp3+ Treg function. Further, provided are high throughput methods for identifying compounds that can inhibit the immunosuppressive function of IL-10 producing Tr1 cells.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Expression of foxp3 by cancer cells
InactiveUS20100143359A1Treat and prevent PTLDReduce expressionOrganic active ingredientsGenetic material ingredientsCancer cellOncology
The present invention relates to the treatment, diagnosis, and prophylaxis of cancer based on the expression of foxp3.
Owner:LUDWIG INST FOR CANCER RES
Methods of enriching and using regulatory t cells
Disclosed are methods of isolating and using a population of FOXP3+ regulatory T cells in a variety of preventative and therapeutic approaches to autoimmune diseases, graft-versus-host disease and transplant rejection.
Owner:UNITED STATES OF AMERICA
Compositions and methods for treating foxp3+ treg related diseases
InactiveUS20130323283A1Increase productionSlow tumor growthBiocidePeptide/protein ingredientsDiseaseTest sample
Methods for treating or preventing a Foxp3+ T regulatory cell (Treg) related disease in a subject in need thereof comprise administering to the subject an effective amount of a pharmaceutical composition comprising an inhibitor of a histone / protein acetyltransferase (HAT). Methods for identifying an agent useful for treating or preventing a Foxp3+ T regulatory cell (Treg) related disease comprise (a) contacting a candidate agent with a test sample comprising Foxp3+ T regulatory cells (Tregs), and (b) comparing a function of the Foxp3+ Tregs in the test sample with that in a control sample, wherein inhibition of the function of the Foxp3+ Tregs in the test sample when compared with the control sample indicates that the candidate agent is an agent useful for treating or preventing a Foxp3+ Treg related disease.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
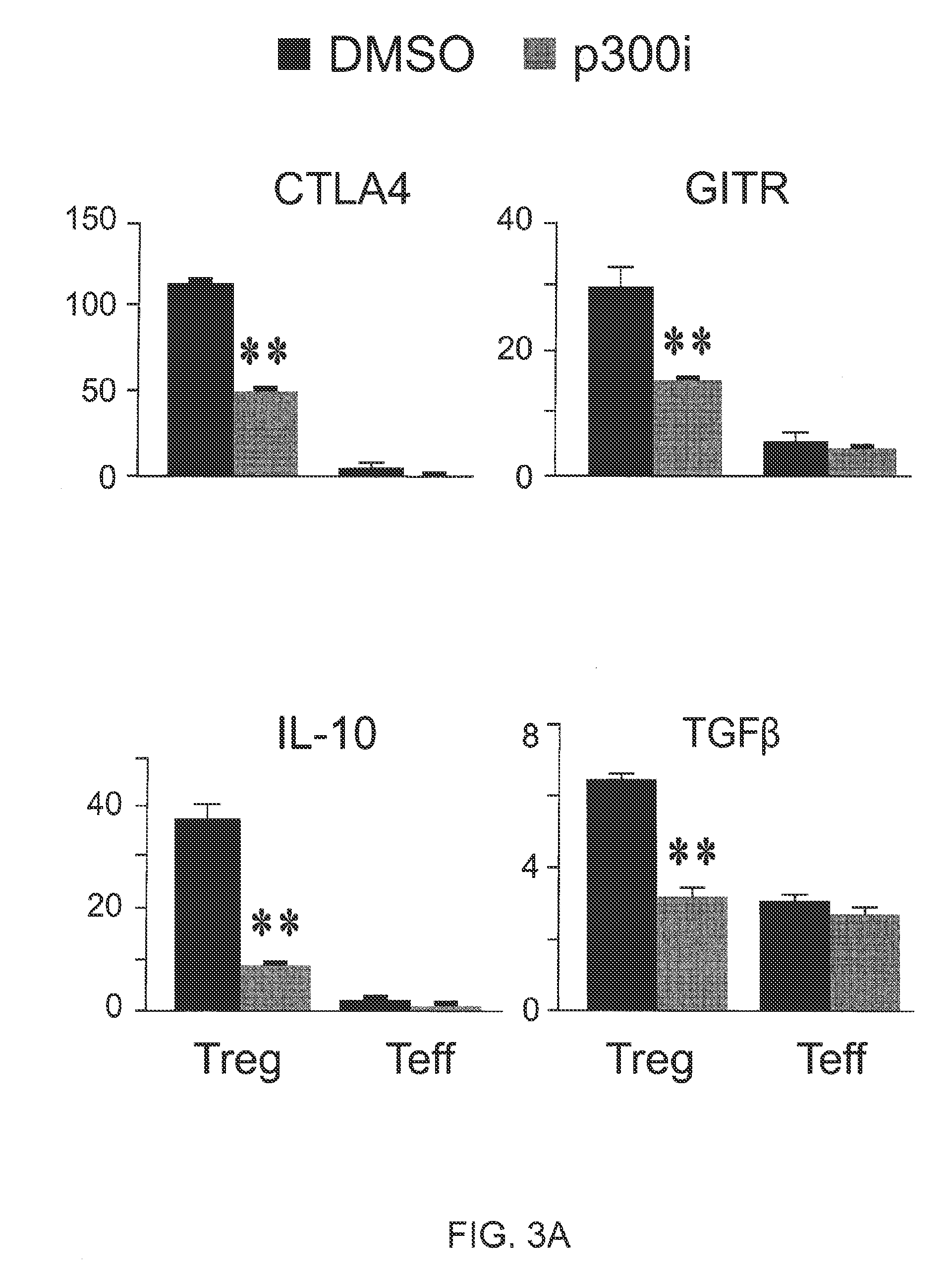
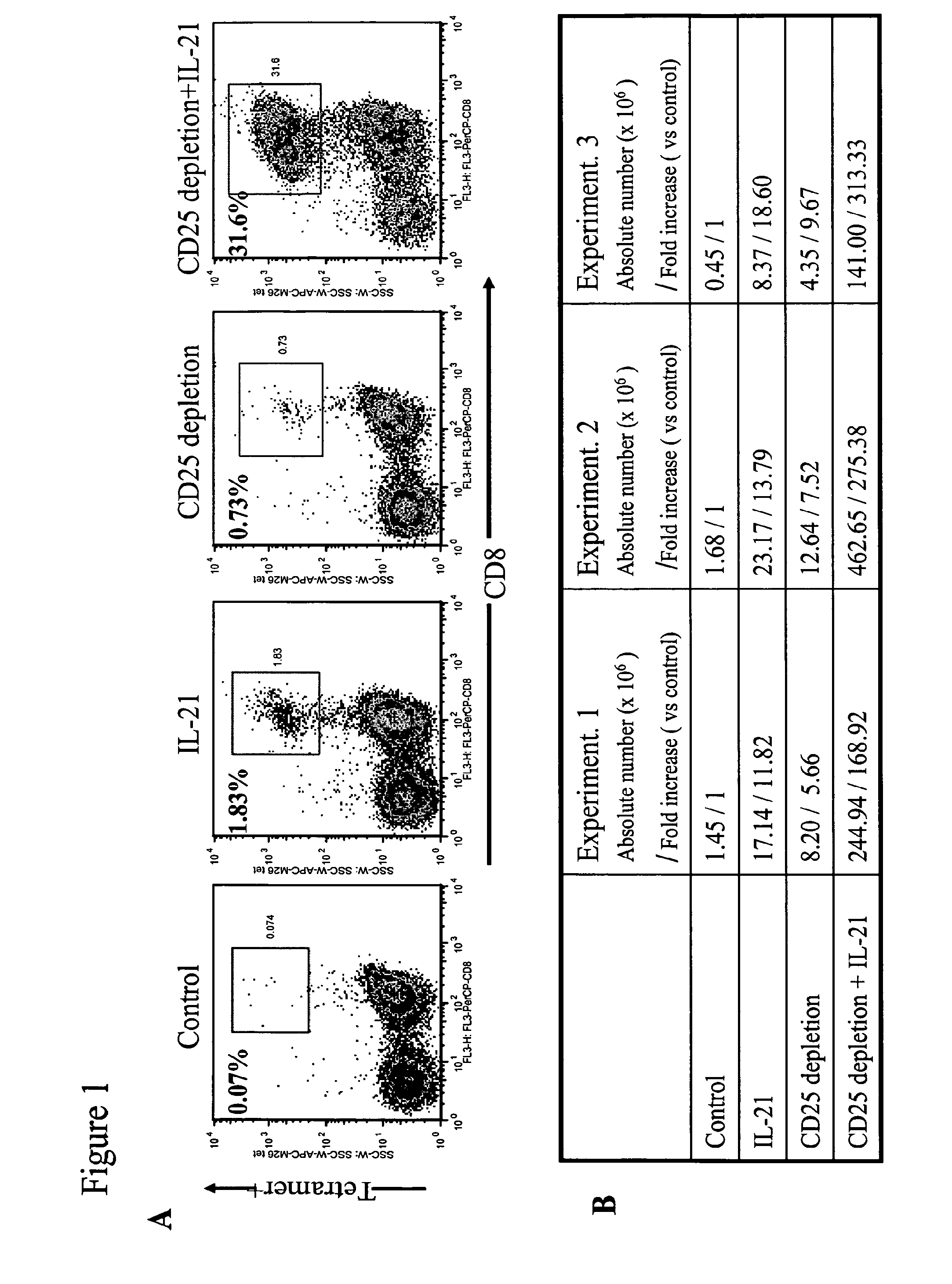
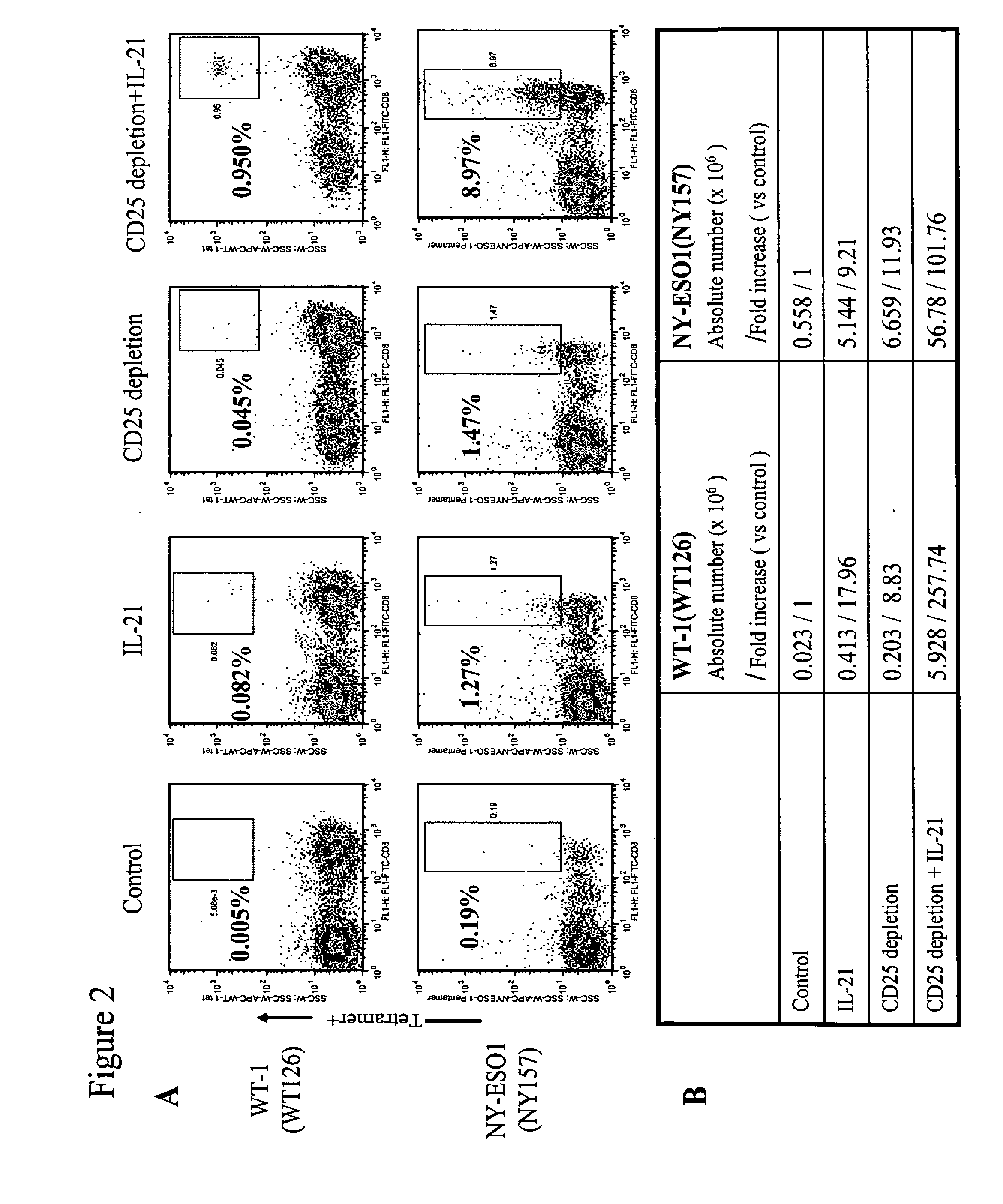
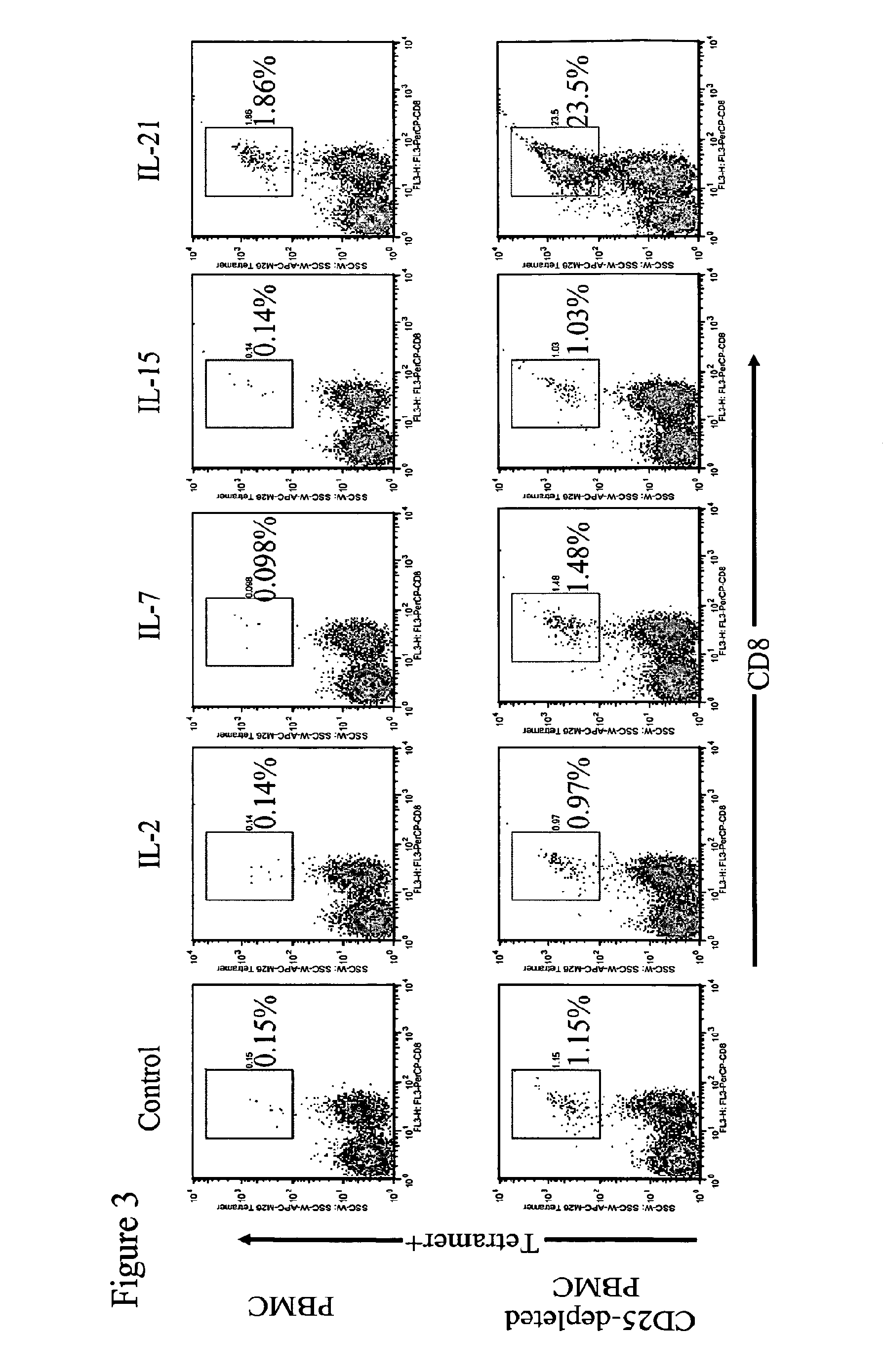
Enhanced generation of cytotoxic t lymphocytes by il-21 mediated foxp3 suppression
ActiveUS20160298081A1Mammal material medical ingredientsBlood/immune system cellsWhite blood cellAntigen stimulation
A method of carrying out adoptive immunotherapy by administering a subject an antigen-specific cytotoxic T lymphocytes (CTL) preparation in a treatment-effective amount is described. In the method, the CTL preparation is preferably administered as a preparation of an in vitro antigen-stimulated and expanded primate CTL population, the CTL population: (i) depleted of FoxP3+ T lymphocytes prior to antigen stimulation; (ii) antigen-stimulated in vitro in the presence of interleukin-21; or (iii) both depleted of FoxP3+ T lymphocytes prior to antigen stimulation and then antigen-stimulated in vitro in the presence of interleukin-21. Methods of preparing such compositions, and compositions useful for carrying out the adoptive immunotherapy, are also described.
Owner:FRED HUTCHINSON CANCER CENT
Epigenetic modification of the loci for CAMTA1 and/or FOXP3 as a marker for cancer treatment
PendingUS20070243161A1Short overall survivalConvenient treatmentOrganic active ingredientsPeptide/protein ingredientsDiseaseRegulatory T cell
The present invention relates to a method, in particular an in vitro method, for pan-cancer diagnostics, comprising identifying the amount and / or proportion of stable regulatory T cells in a patient suspected of having cancer through analyzing the methylation status of at least one CpG position in the gene foxp3 and / or the gene camta1 or orthologous or paralogous genes thereof, wherein an increased amount and / or proportion of stable regulatory T cells in said patient is indicative for an unspecific cancerous disease. In a second aspect thereof, the present invention relates to a method for diagnosing the survival of a cancer patient, comprising identifying the amount and / or proportion of stable regulatory T cells in said cancer patient through analyzing the methylation status of at least one CpG position in the gene foxp3 and / or the gene camta1 or orthologous or paralogous genes thereof, wherein a demethylation in the gene foxp3 and / or the gene camta1 or orthologous or paralogous genes thereof, is indicative of a stable regulatory T cell, and wherein an increased amount and / or proportion of stable regulatory T cells in said cancer patient is indicative for a shorter survival for said cancer patient. Furthermore, the present invention relates to an improved treatment of cancers based on the inventive methods, and a kit for performing the above methods as well as respective uses.
Owner:PRECISION FOR MEDICINE GMBH
Assays for detecting t cell immune subsets and methods of use thereof
InactiveUS20160161485A1Improved prognosisImprove survivalPeptide/protein ingredientsMicrobiological testing/measurementCancer cellFoxp3 expression
The present disclosure provides methods for measuring the number of CD4+ OX40+ Foxp3+ lymphocytes in a sample containing cancer cells and lymphocytes obtained from a subject by labeling lymphocytes that show CD4 expression in the sample, then labeling lymphocytes that show OX40 expression in the sample, then labeling lymphocytes that show Foxp3 expression in the sample, then measuring the number of CD4+ OX40+ Foxp3+ lymphocytes in the sample. Further provided are methods for determining the prognosis of a subject, predicting responsiveness of a subject having cancer to an OX40 agonist treatment, and methods for treating or delaying progression of cancer based on the number of CD4+ OX40+ Foxp3+ lymphocytes in a sample.
Owner:GENENTECH INC
Method for detecting immunosuppression function of human regulatory T cells
InactiveCN106932576AStrong immunosuppressive functionBiological material analysisIndividual particle analysisRegulatory T cellCell Surface Antigens
The invention discloses a method for detecting an immunosuppression function of human regulatory T cells. The method comprises the following steps: collecting peripheral blood for extracting a mononuclear cell; using fluorescein marked anti-CD4 and anti-CD25 antibodies for marking a cell surface antigen; using a fixing membrane-breaking agent for fixing the cell surface antigen; after breaking the membrane, using fluorescein marked anti-Foxp3 and anti-Helios antibodies for marking endonuclear transcription factor Foxp3 and Helios; and finally, using a flow cytometry for detecting CD4+CD25+FoxP3+Helios+Treg cell population. According to the method, the function of the regulatory T cells in the peripheral blood is directly analyzed in the manner of quickly detecting the expression condition of the children peripheral blood transcription factor Helios. According to the method, the anti-Helios, anti-CD4, anti-CD25 and anti-Foxp3 flow antibodies can be used for more quickly and accurately marking the peripheral blood Treg cells with higher immunosuppression function.
Owner:山东大学深圳研究院
Methods of switching the phenotype of t cells by transgenic lineage factor foxp3
InactiveUS20100203068A1Conveniently removedReduce needGenetically modified cellsSnake antigen ingredientsGenetic elementT cell
In one aspect the invention relates to a method of switching the phenotype of a target cell, said method comprising inducing lineage factor activity in said cell via a transgene. In another aspect, the invention relates to a method of switching the phenotype of a target cell, said method comprising introducing to said cell a genetic element capable of inducibly generating lineage factor activity, and inducing lineage factor activity in said cell. The invention also relates to methods of suppressing immune responses and methods of treating subjects.
Owner:MEDICAL RESEARCH COUNCIL
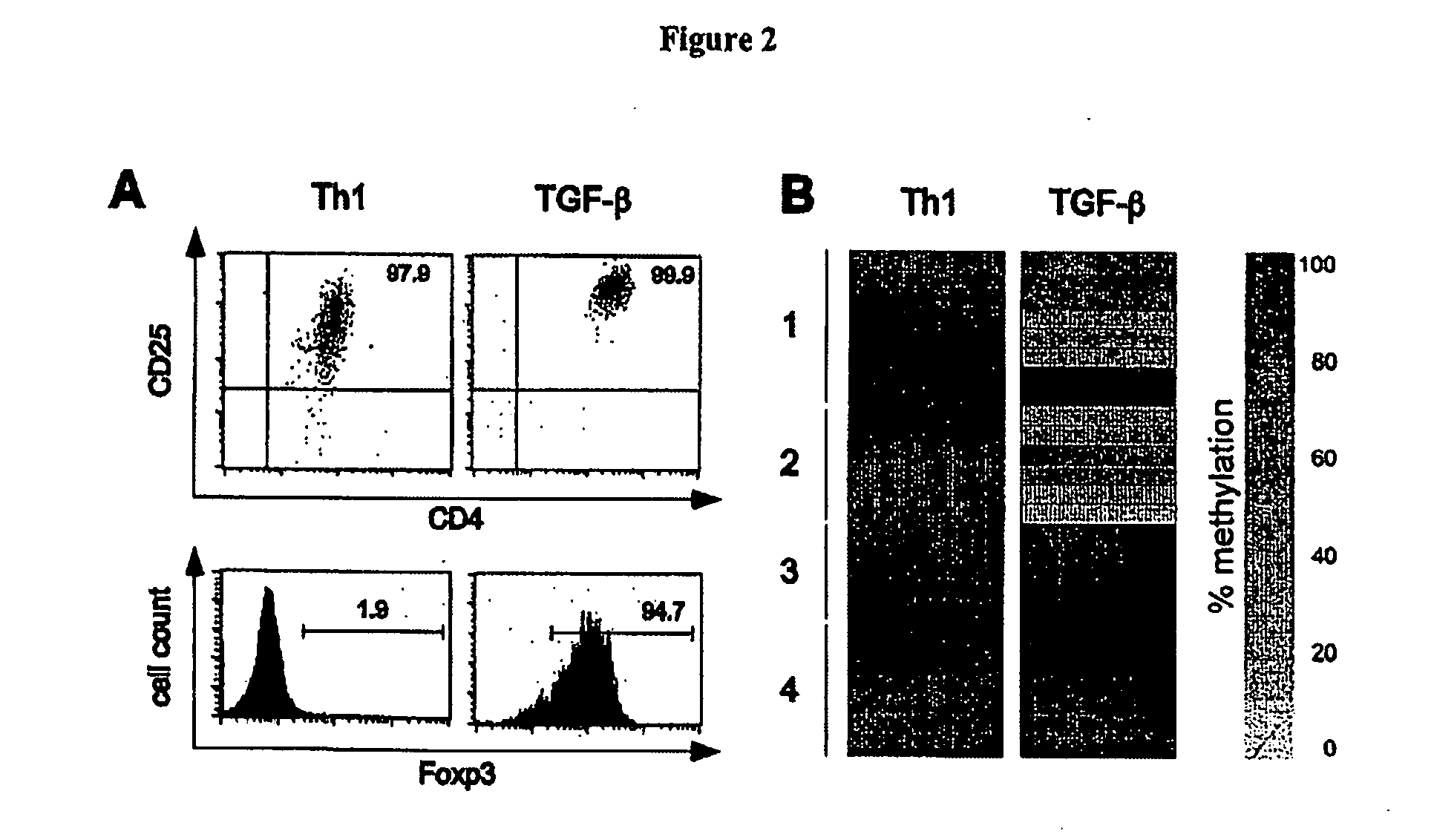
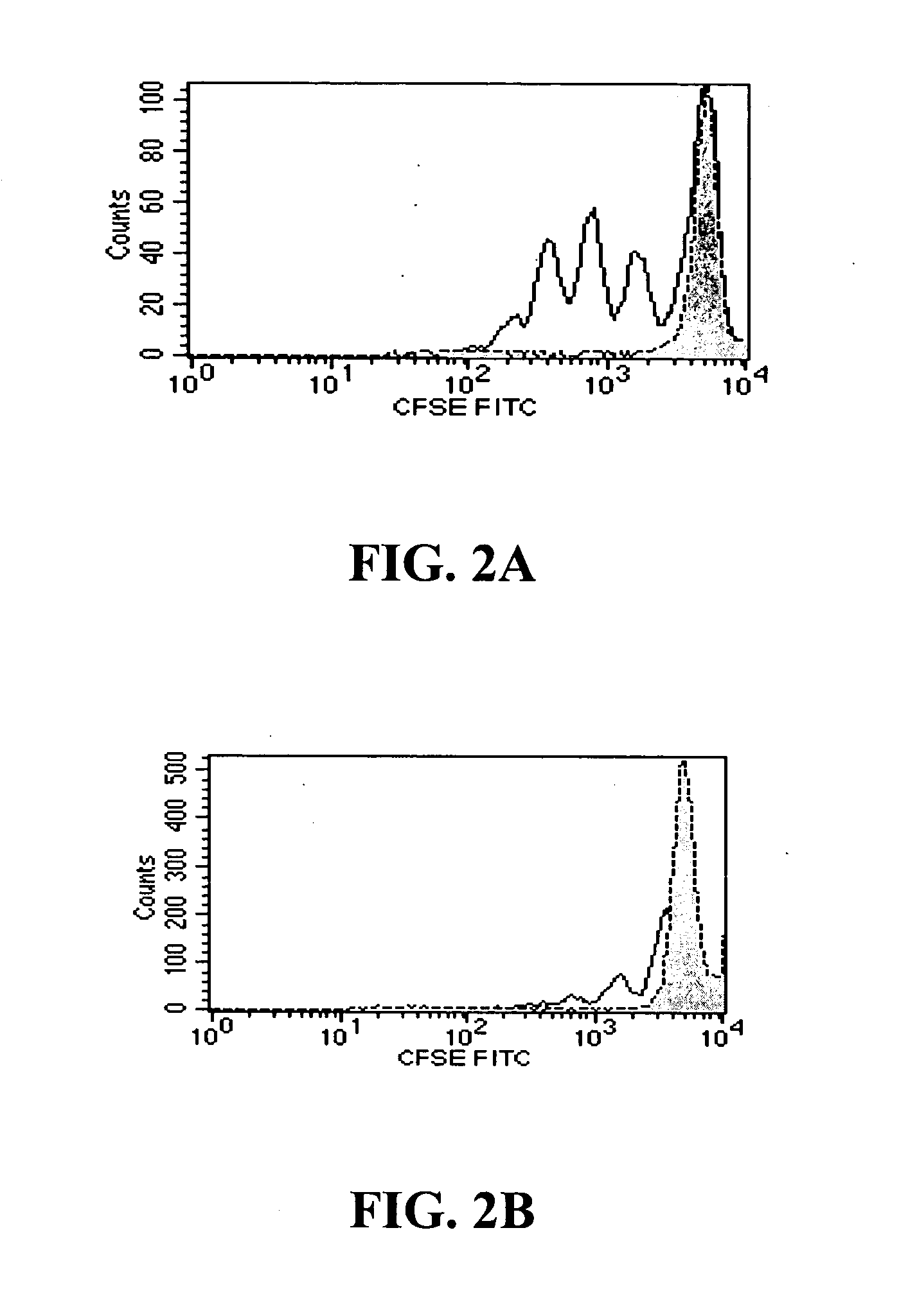
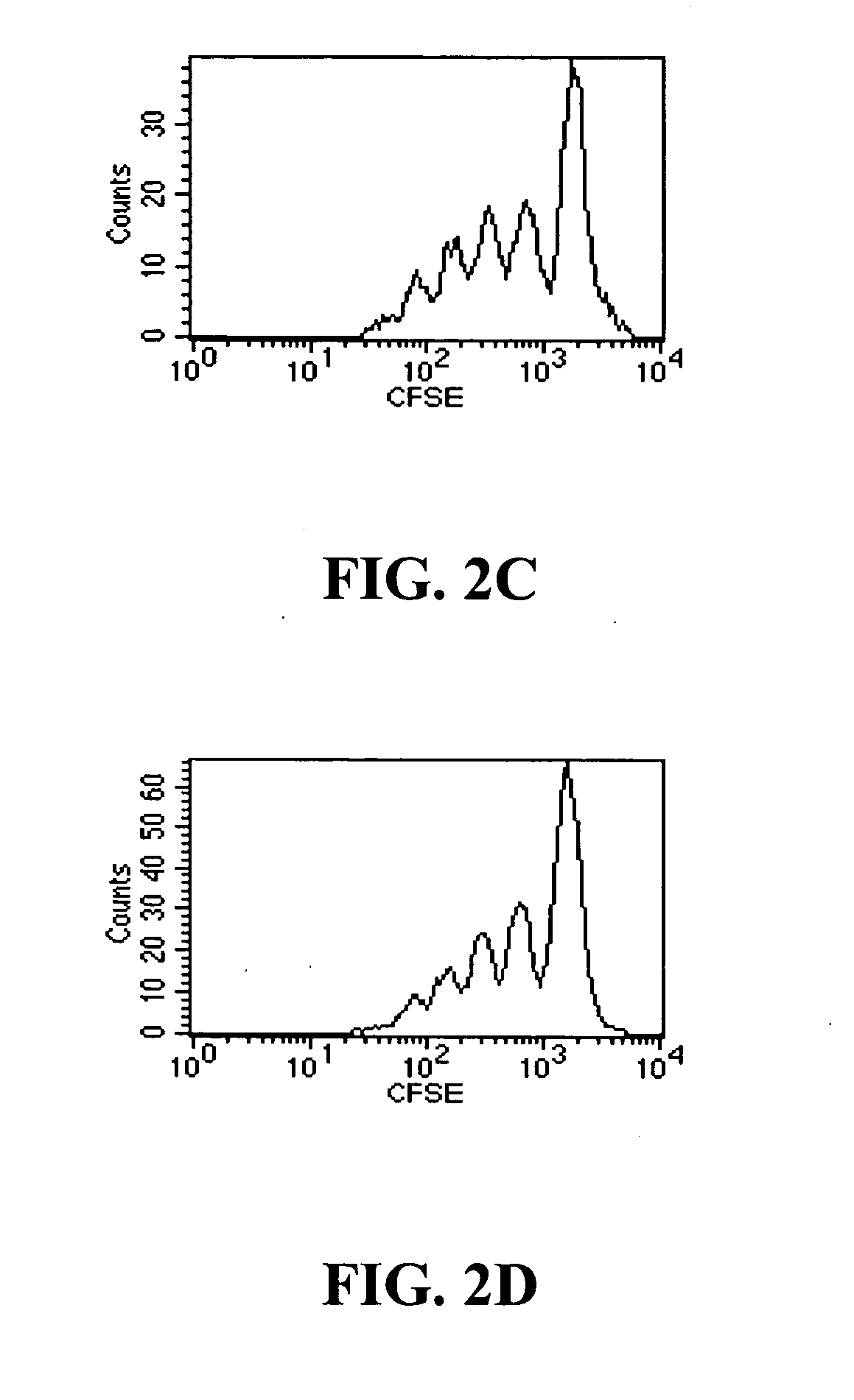
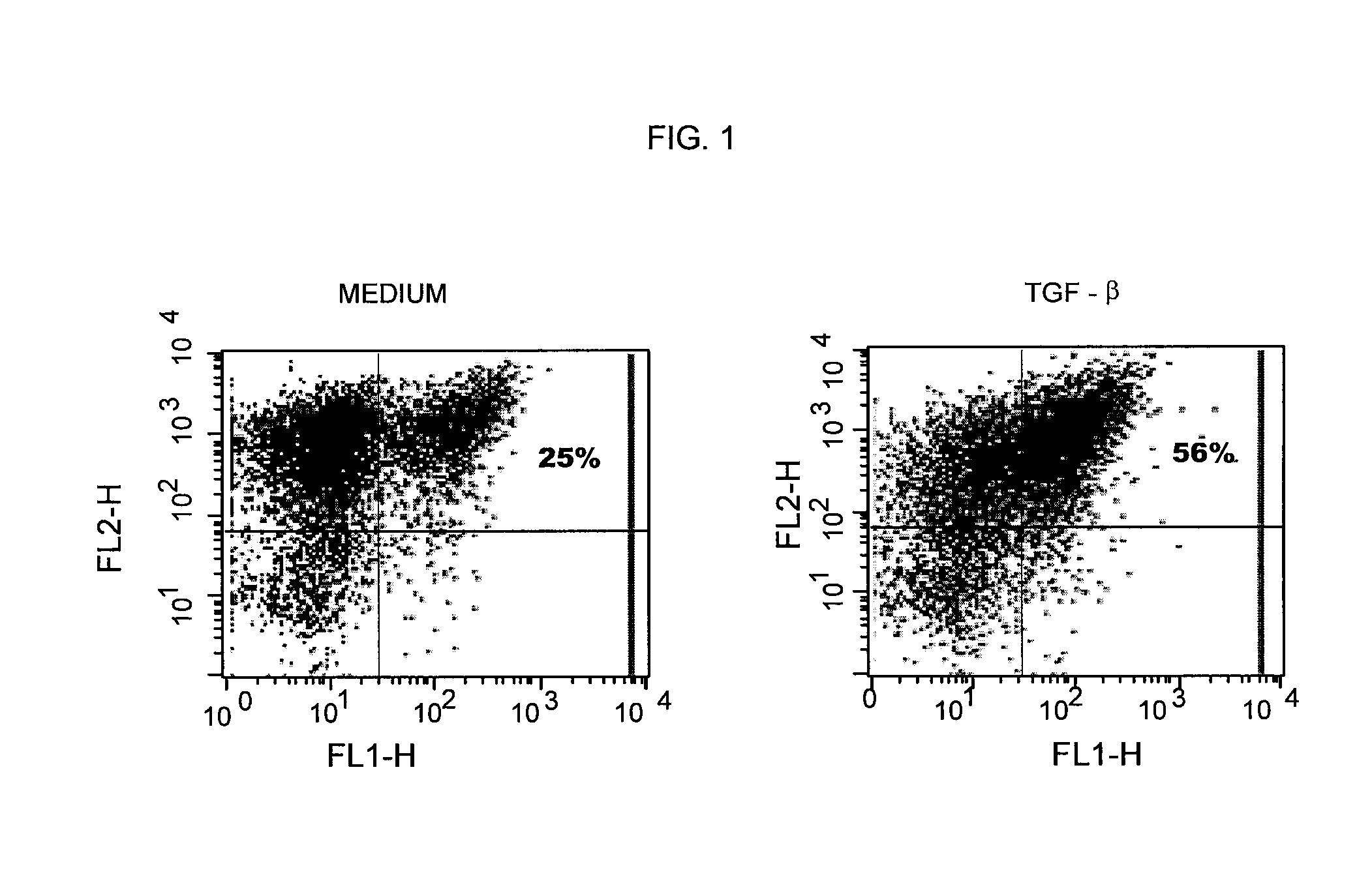
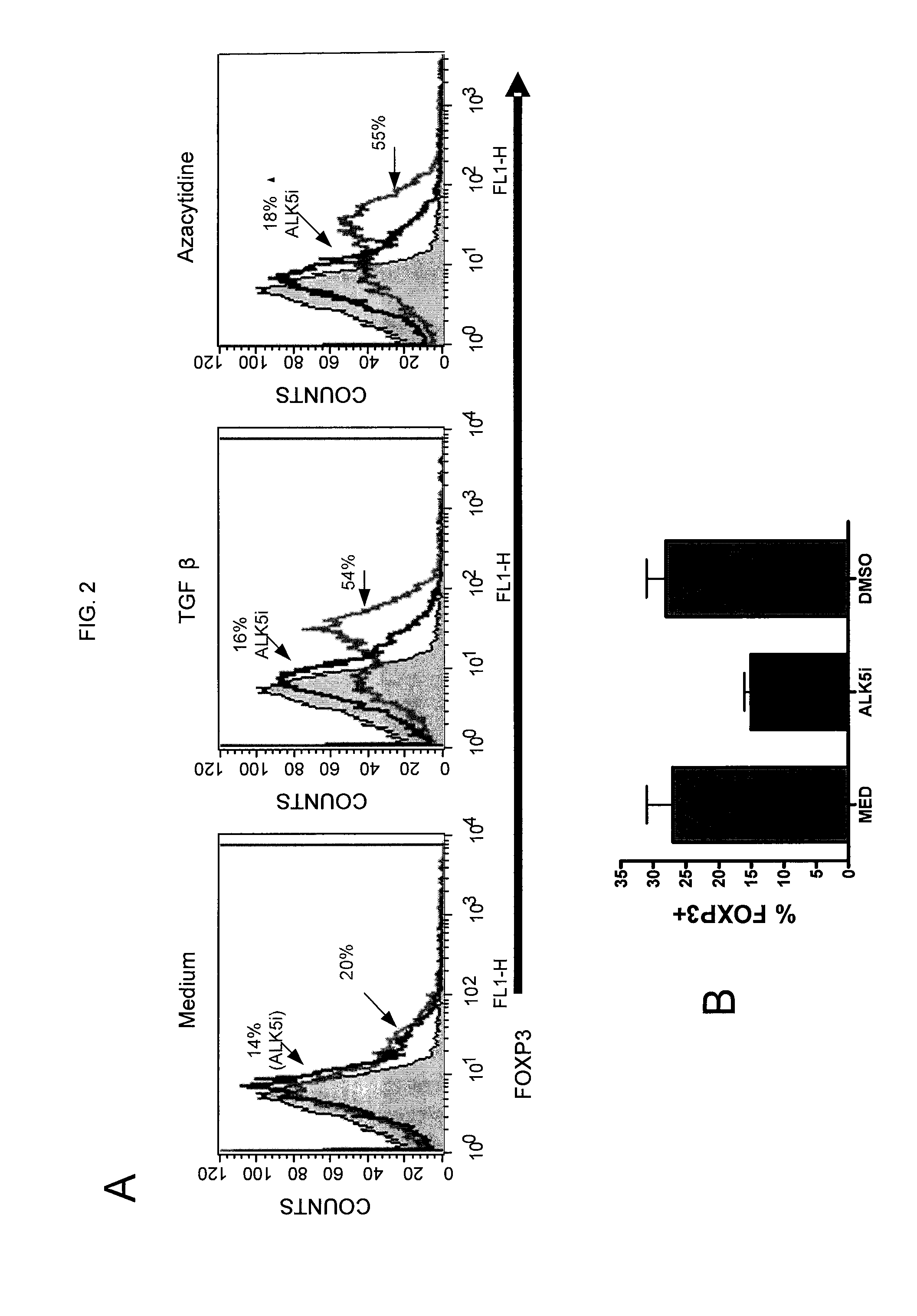
TGF-beta induced adjustment T cell and its forming method and application
InactiveCN101126076APrevent proliferationInhibitionMammal material medical ingredientsBlood/immune system cellsDiseaseRegulatory T cell
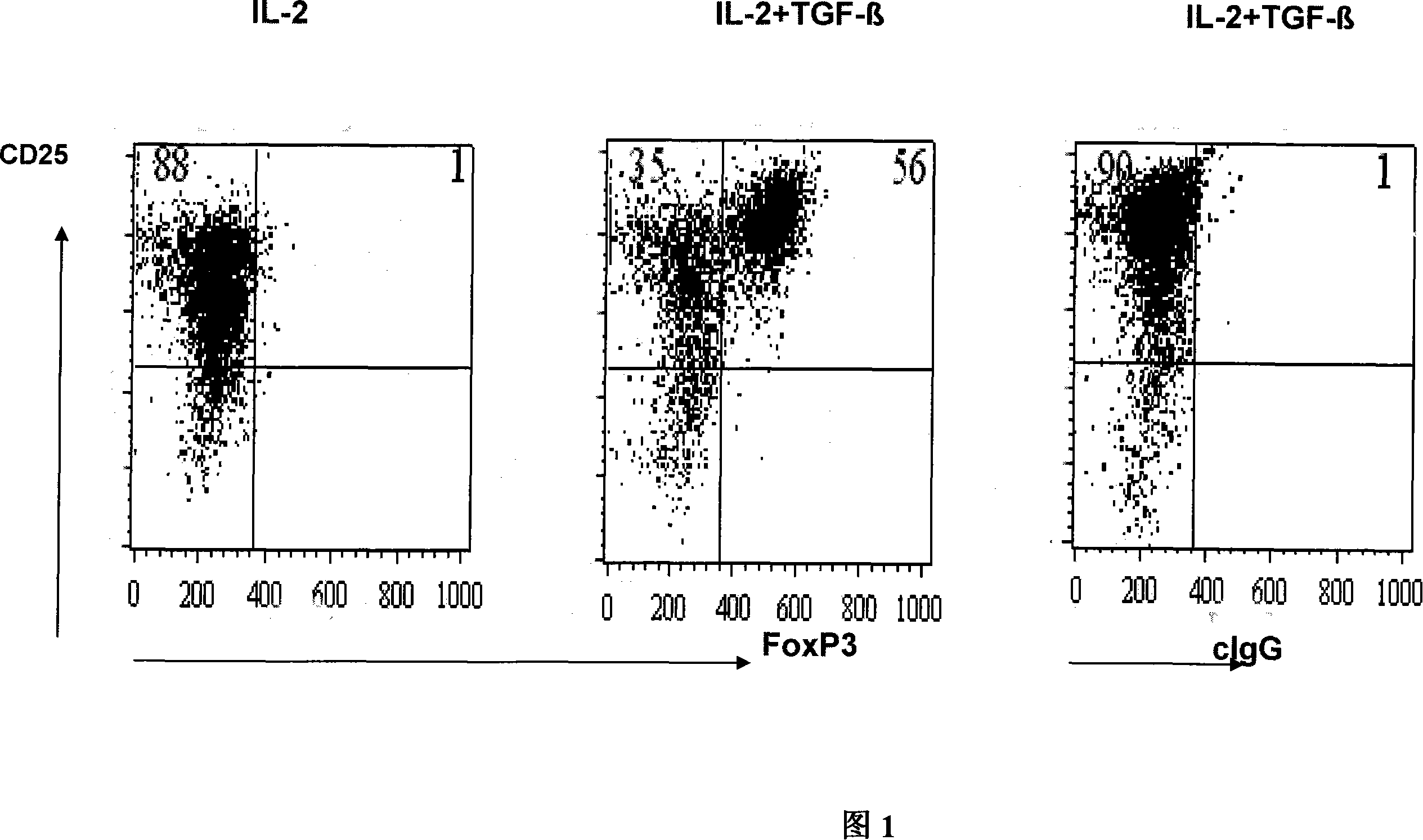
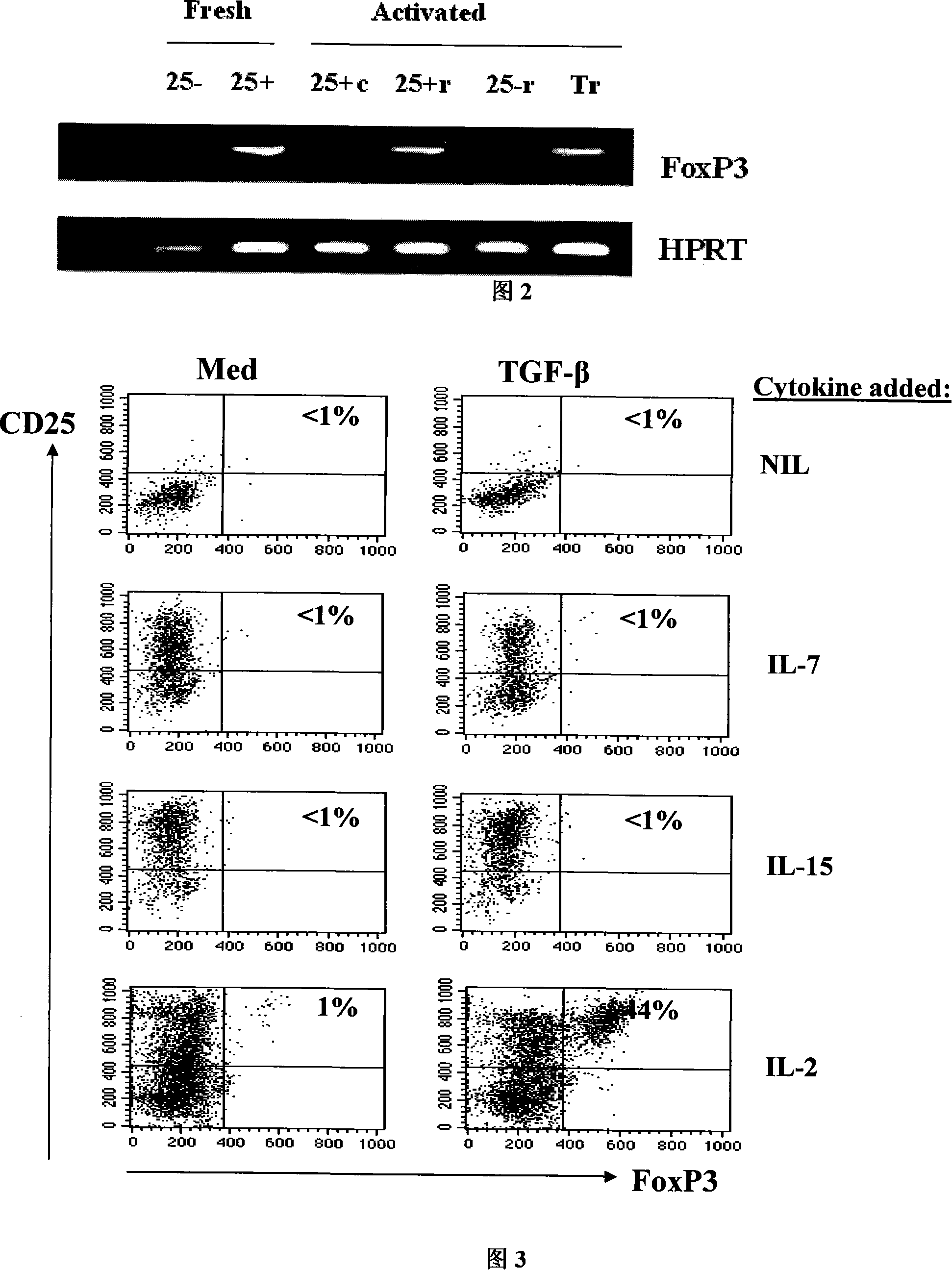
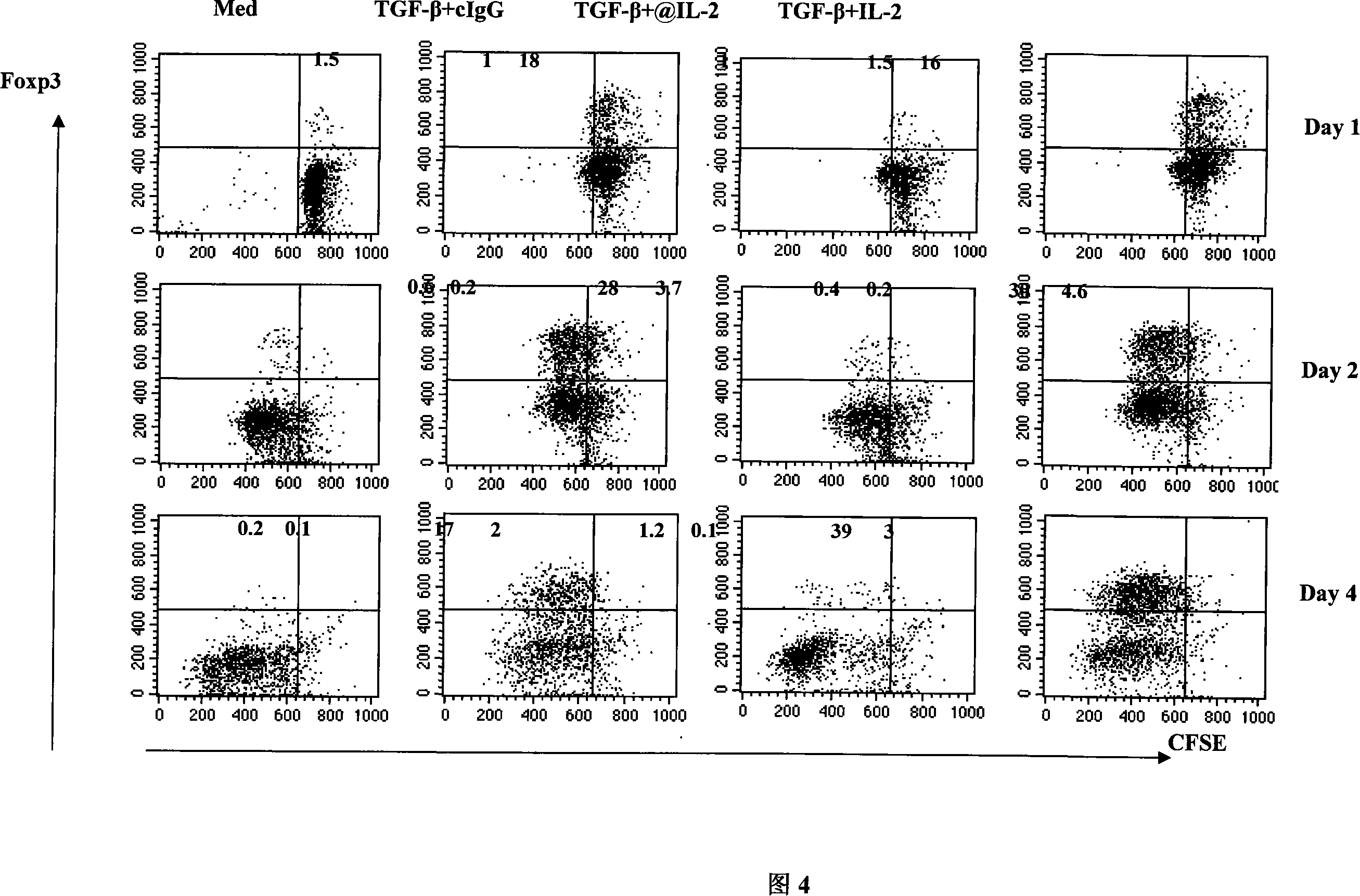
The invention pertains to biomedical technique field, in particular to a TGF-beta induced regulatory T-cell and a synthesis method and the applications thereof. The regulatory T-cell of the invention is formed by combining two cytokines: TGF-Beta and IL-2, and obtained by extrinsically inducing CD4+ cells of autoimmune diseases sufferer, and is recorded as CD4++CD25+Foxp3+ regulatory T-cell, wherein FoxP3 is FoxP3 gene or protein. The regulatory T-cell of the invention can be taken as a medicine preparation that is used for treating the diseases of autoimmune diseases (such as SLE) sufferer.
Owner:郑颂国 +4
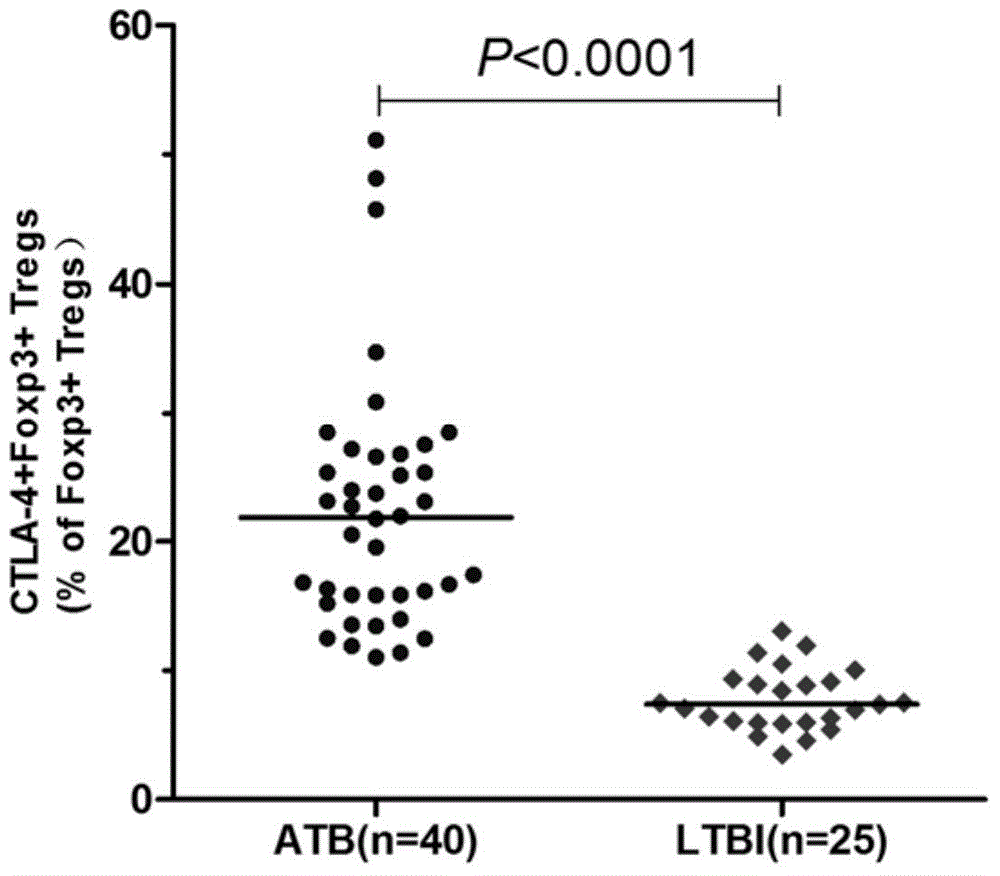
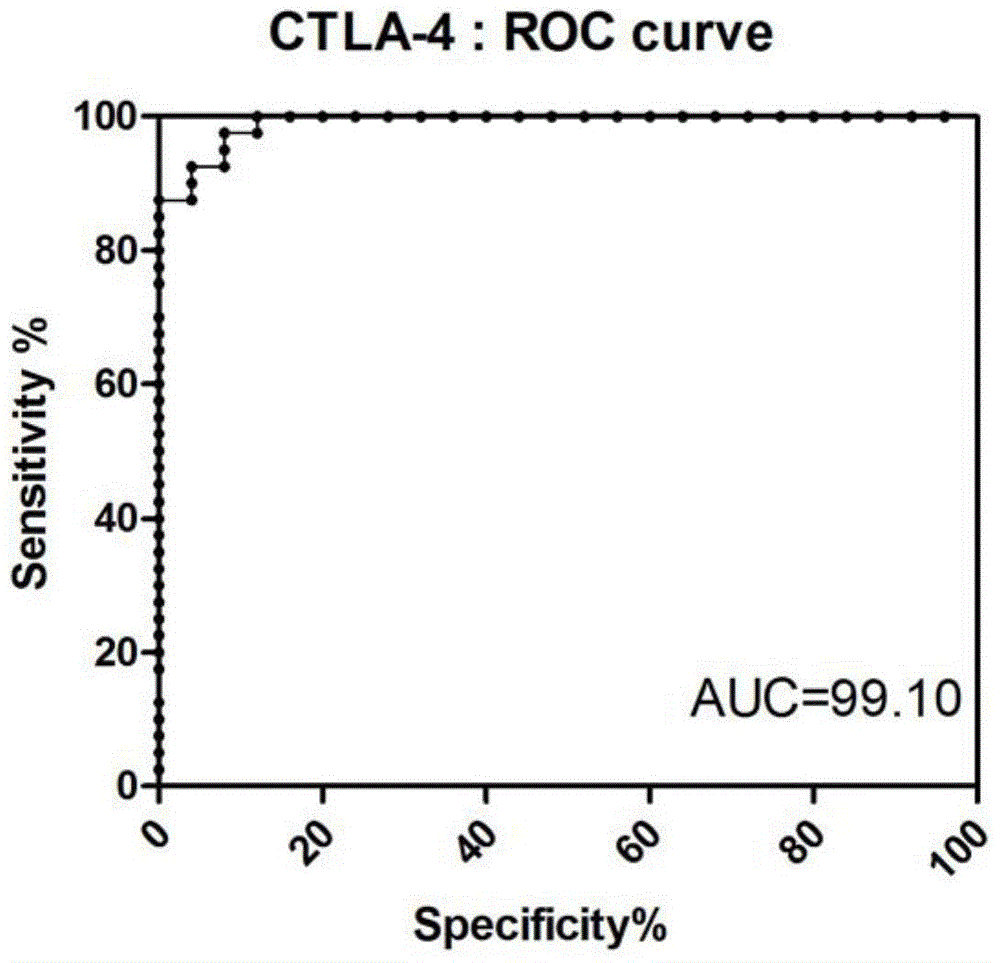
Diagnostic kit for distinguishing active and latent mycobacterium tuberculosis infection
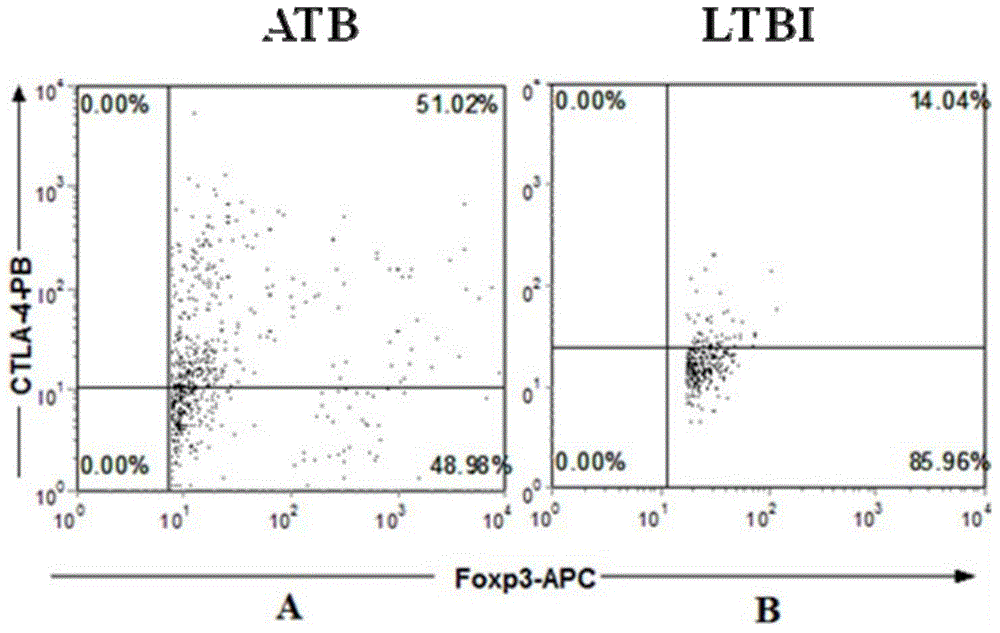
The invention provides a diagnostic kit for distinguishing active and latent mycobacterium tuberculosis infection with cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) serving as a diagnosis marker and a method for distinguishing active and latent mycobacterium tuberculosis infection by means of the diagnostic kit. The diagnostic kit comprises a CTLA-4, CD3, CD4, CD25, FoxP3 fluorescent antibody, erythrocyte lysate, membrane breaking liquid, washing buffer, phosphate buffer, fetal calf serum, stationary liquid and a streaming pipe. The diagnostic kit can be used for detecting the CTLA-4 expression quantity of peripheral venous blood of a patient and distinguishing active and latent mycobacterium tuberculosis infection, sensitivity and accuracy are high, and a strong basis is provided for clinic differential diagnosis.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
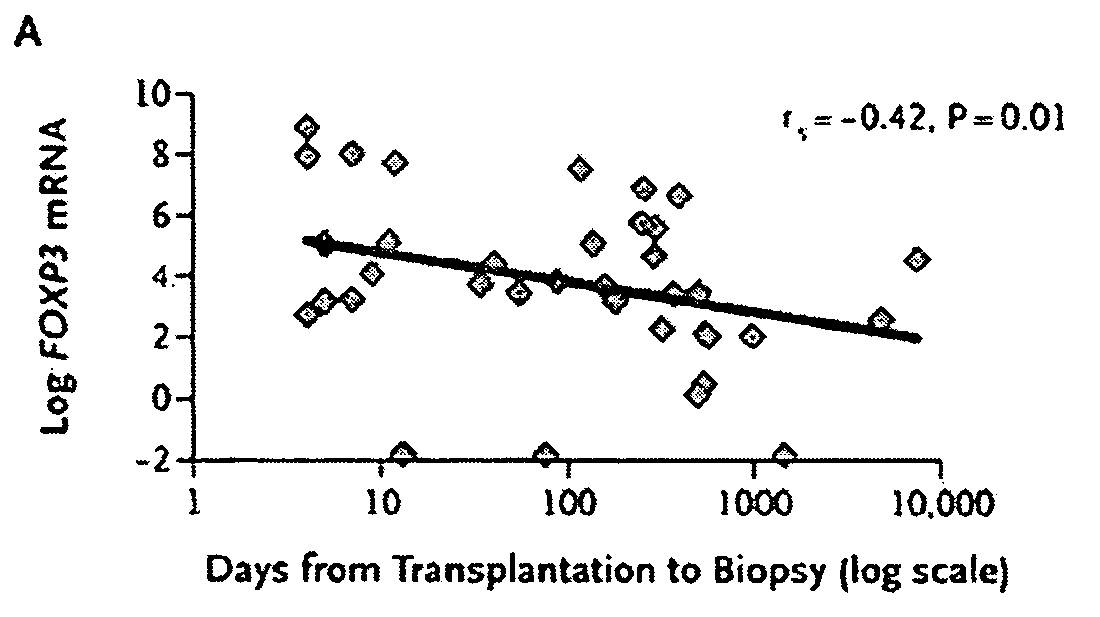
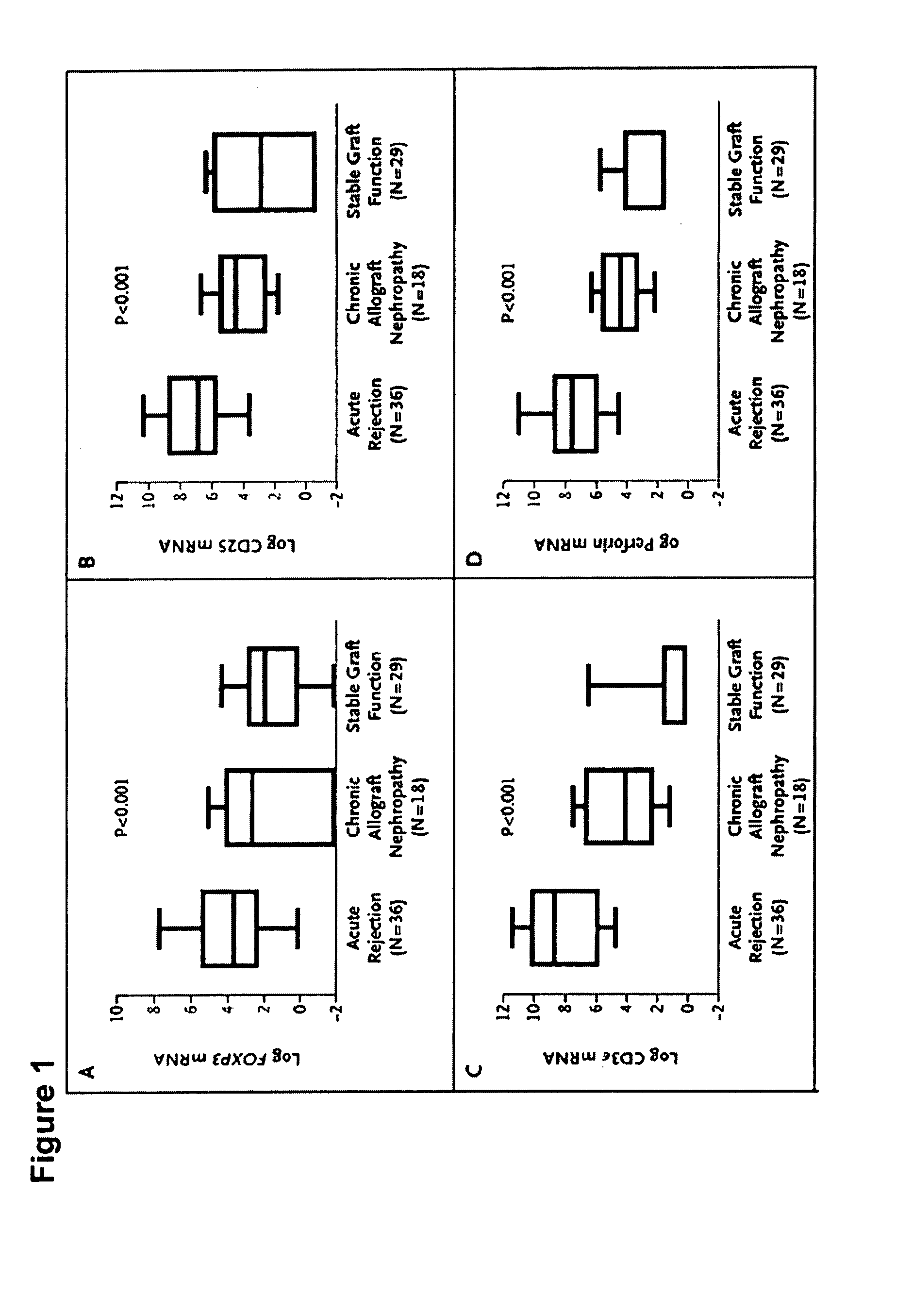
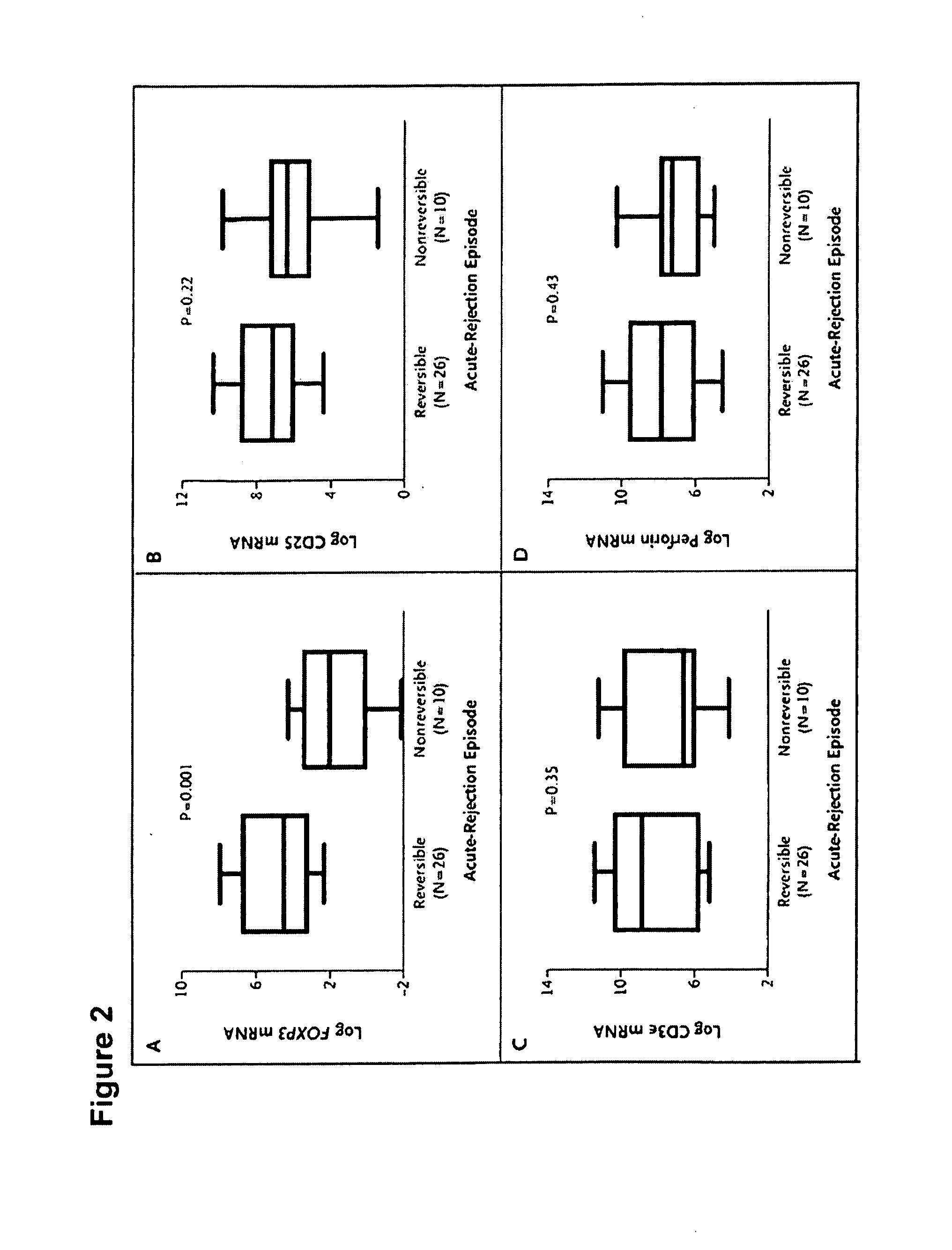
Methods of Using FOXP3 Levels to Predict the Outcome of Organs Undergoing Acute Rejection
InactiveUS20080131441A1Improve the level ofReduce riskOrganic active ingredientsMicrobiological testing/measurementSurgeryTransplanted Organs
A method for assessing risk of losing a transplanted organ by a patient having an episode of acute rejection of the transplanted organ is described. The method includes obtaining from the patient a cell sample from the transplanted organ or peripheral blood, determining a level of FOXP3 in the cell sample, and correlating the level with the risk of loss of the transplanted organ, wherein, compared to a control level, a significantly greater level of FOXP3 in the cell sample from the transplanted organ or a significantly lower level of FOXP3 in the cell sample from the peripheral blood correlates with a decreased risk of loss of the transplanted organ.
Owner:CORNELL RES FOUNDATION INC
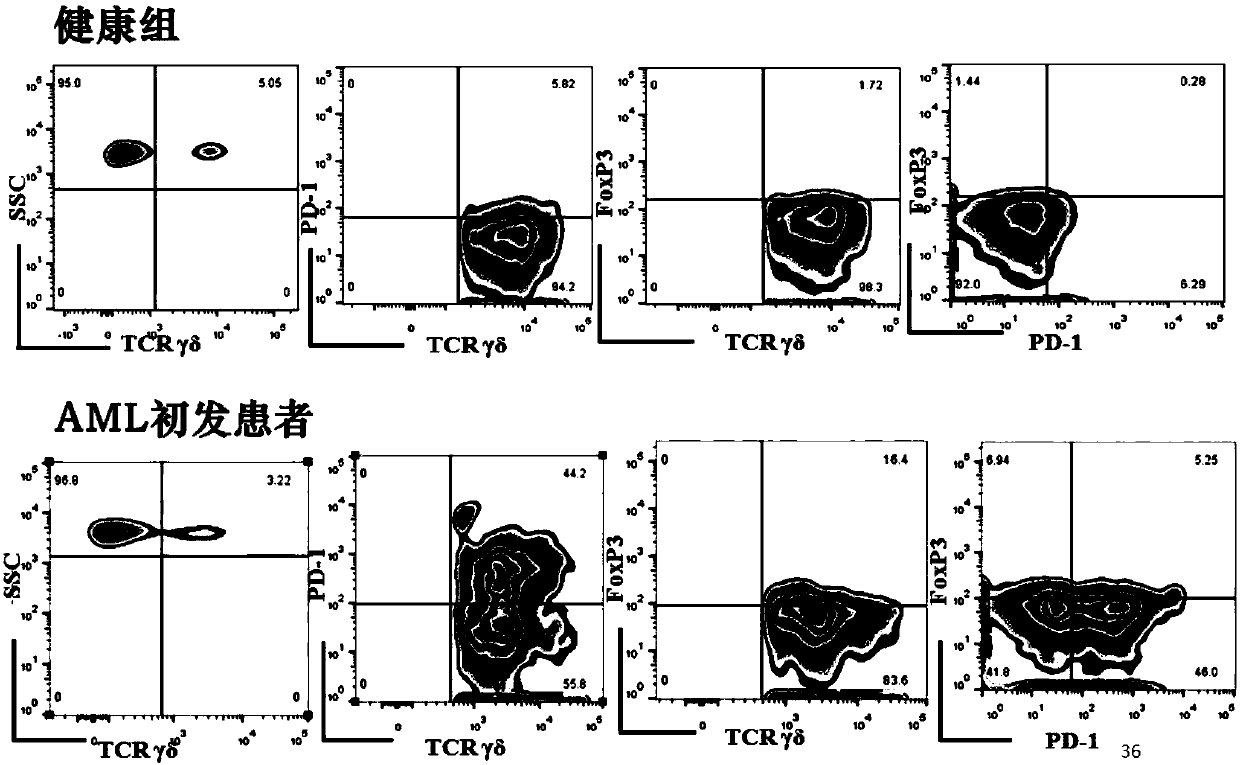
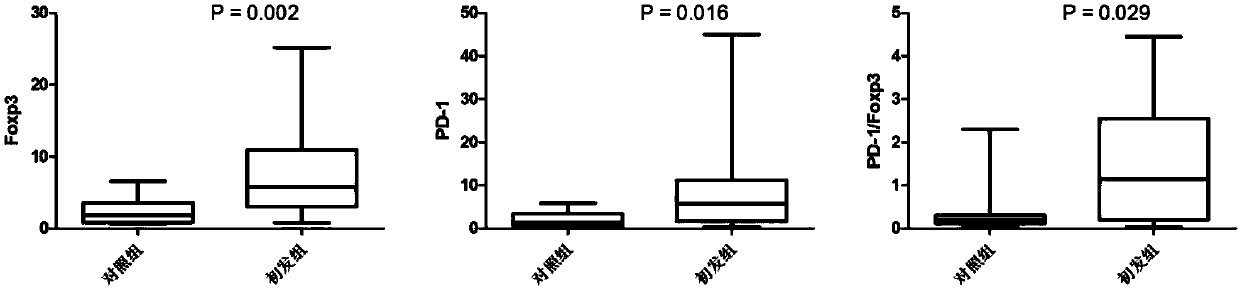
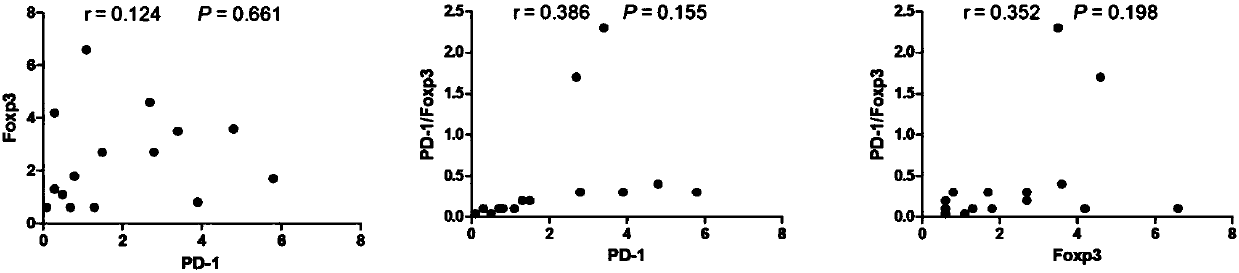
Application of novel gamma delta T cell to preparation of kit for evaluating curative effect of AML (acute myeloid leukemia)
ActiveCN107860924APredict clinical responsePredictive prognostic evaluationDisease diagnosisMyeloid leukemiaClinical efficacy
The invention provides application of a novel gamma delta T cell subpopulation to preparation of a kit for predicting the curative effect and prognosis of AML (acute myeloid leukemia). The inventor based on the invention discovers the expression condition of the novel gamma delta T cell subpopulation in peripheral blood of patients suffering from AML is associated with the curative effect and theprognosis of the patients suffering from AML for the first time. When the expression ratio of the novel PD1+Foxp3+gamma delta T cell subpopulation is high, the possibility of bad clinical curative effect of the patients suffering from AML is high. The expression ratio of the novel gamma delta T cell subpopulation has important guide significance in prognosis judgment of the patients suffering fromAML and formulation of a clinical treatment scheme. More base research data can be provided for individual treatment of the patients suffering from AML, and wide application prospect in clinical curative effect and prognosis evaluation of the patients suffering from AML is achieved.
Owner:上海普锐暨医学检验实验室有限公司
Method for identifying antigen-specific regulatory t cells
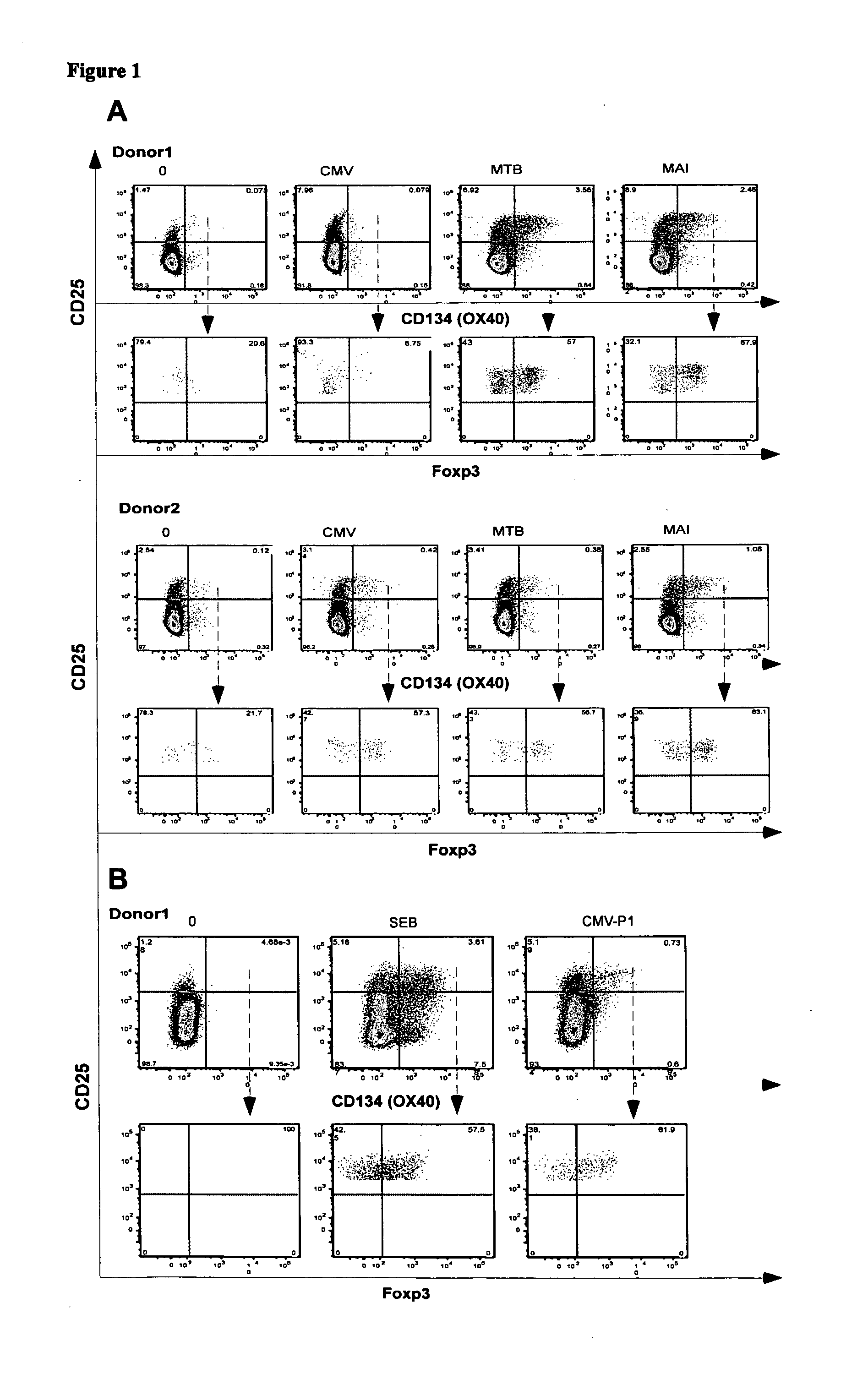
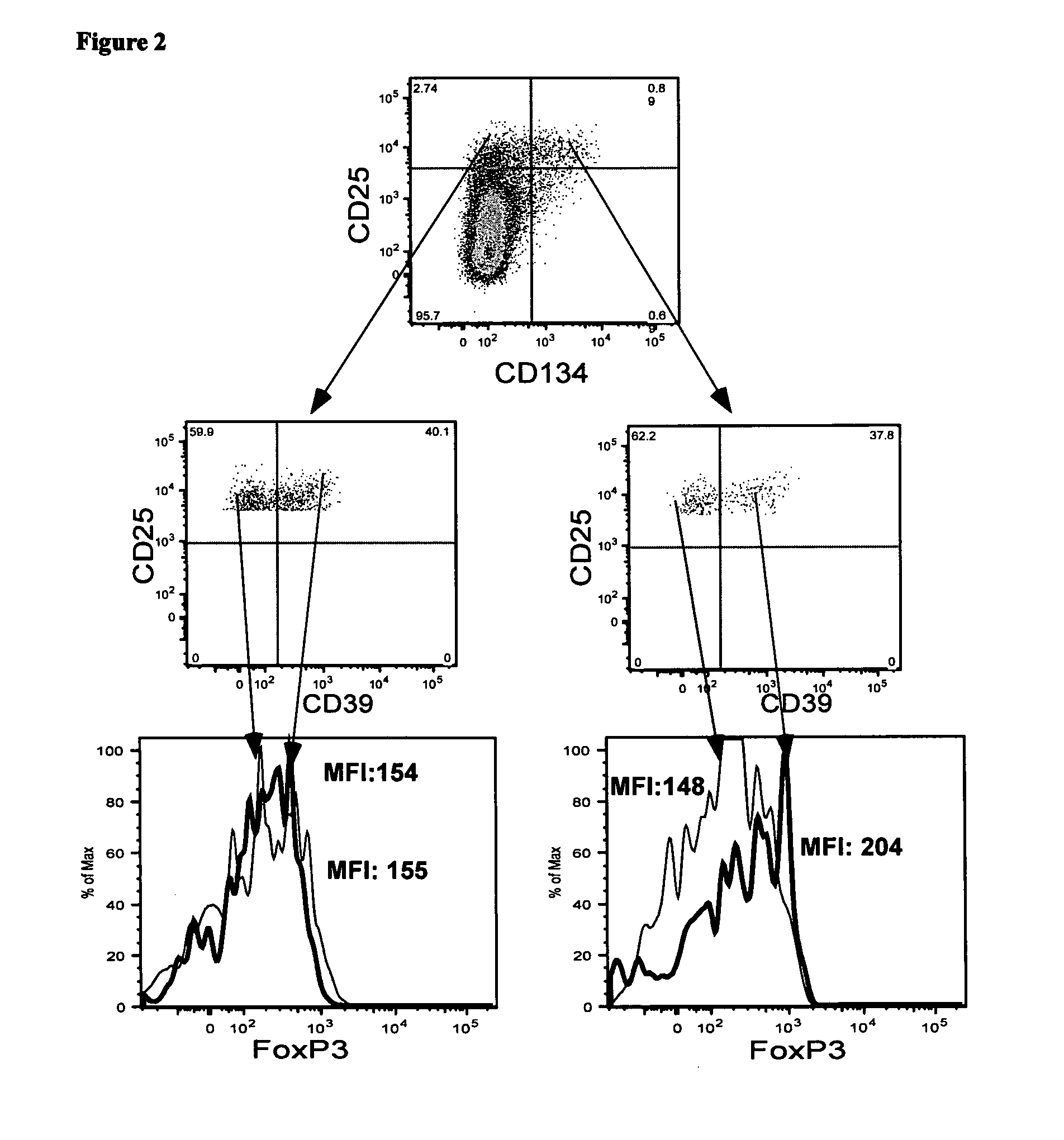
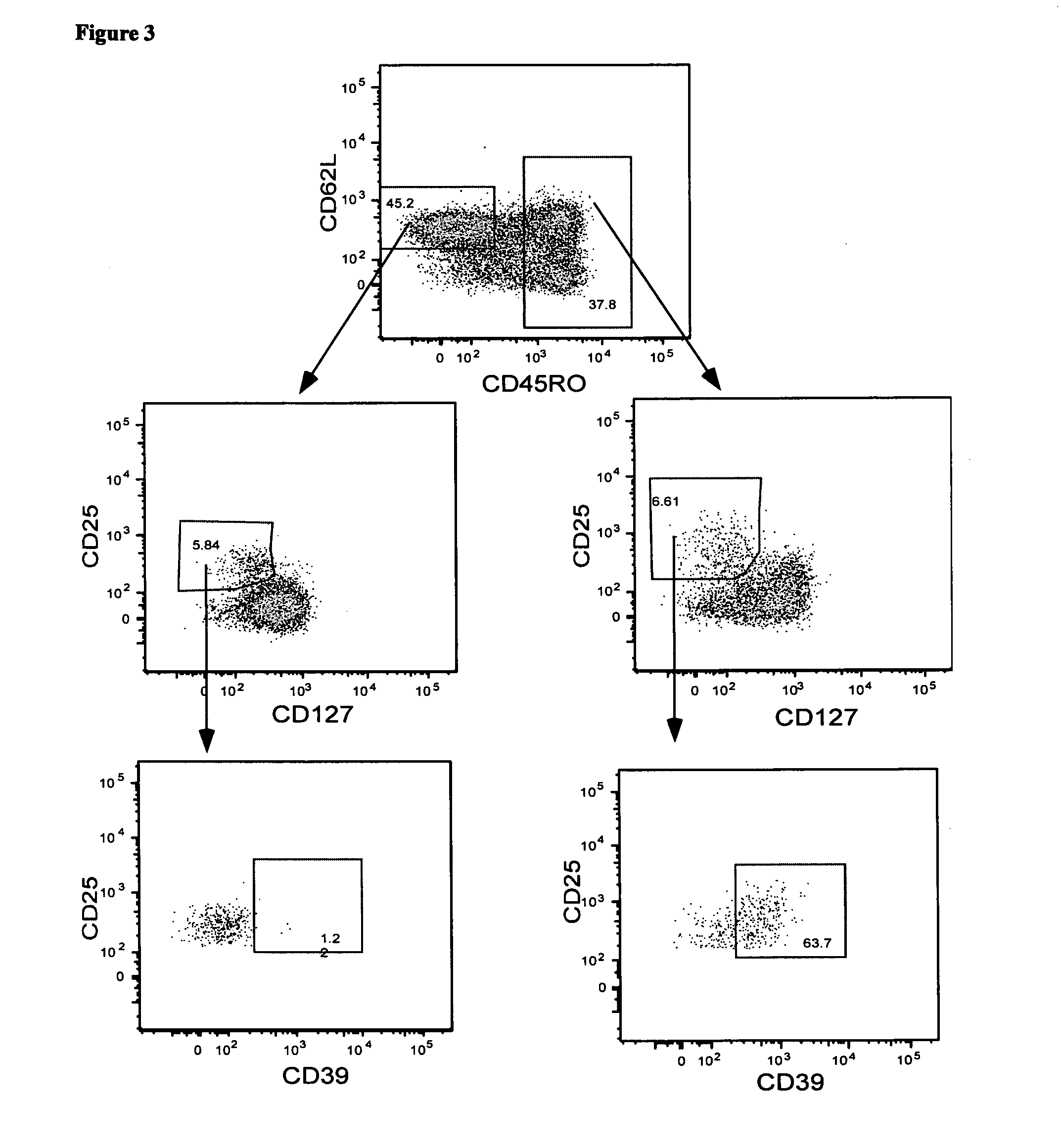
A method of identifying an antigen-specific regulatory T cell (Treg) from a subject is discussed wherein the method comprises quantitatively or qualitatively detecting co-expression of each of cell markers CD4, CD25 and CD134, or alternatively, N each of cell markers CD8, CD25 and CD137, as well as one or more cell markers selected from the group of Treg cell markers consisting of CD39, CD73, CD127, CTLA-4 and Foxp3 on a cell in a suitable lymphocyte-containing sample from the subject in response to exposure to a target antigen. Also discussed are methods of isolating and expanding the identified antigen-specific Treg population, which may permit antigen-specific Treg cell therapy.
Owner:ST VINCENTS HOSPITAL SYDNEY
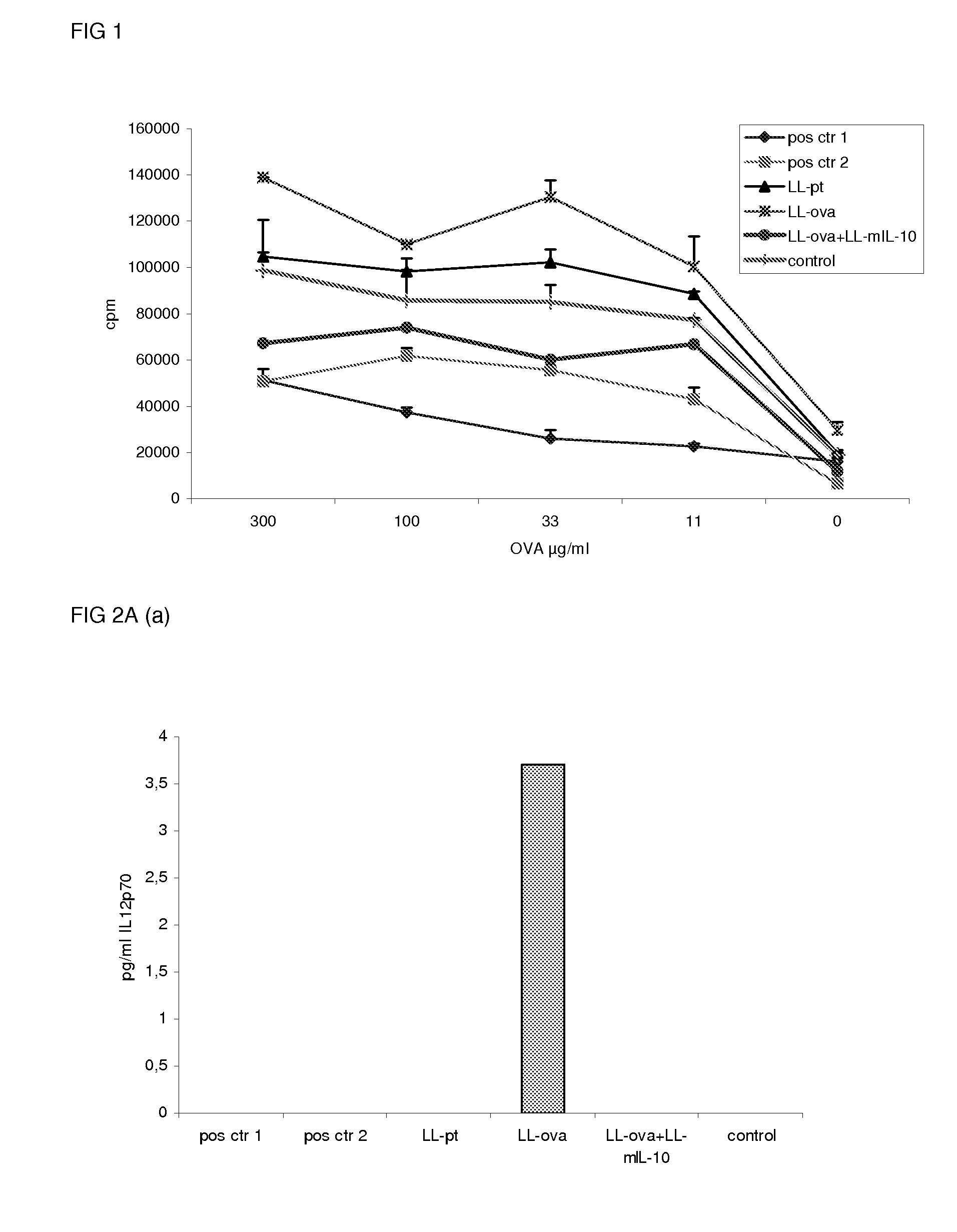
Induction of mucosal tolerance to antigens
ActiveUS20090148389A1Enhanced inhibitory effectStable responsePeptide/protein ingredientsAerosol deliveryMicroorganismAntigen
Induction of tolerance to antigens by mucosal, preferably oral, delivery of the antigen in combination with an immunomodulating compound producing micro-organism is disclosed. More specifically, Foxp3+ and / or IL-10 and / or TGF-β producing regulatory T-cells are induced which are capable of suppressing undesired immune responses toward an antigen. The antigen is preferably delivered orally in combination with an immunosuppressing cytokine secreting micro-organism.
Owner:INTREXON ACTOBIOTICS NV
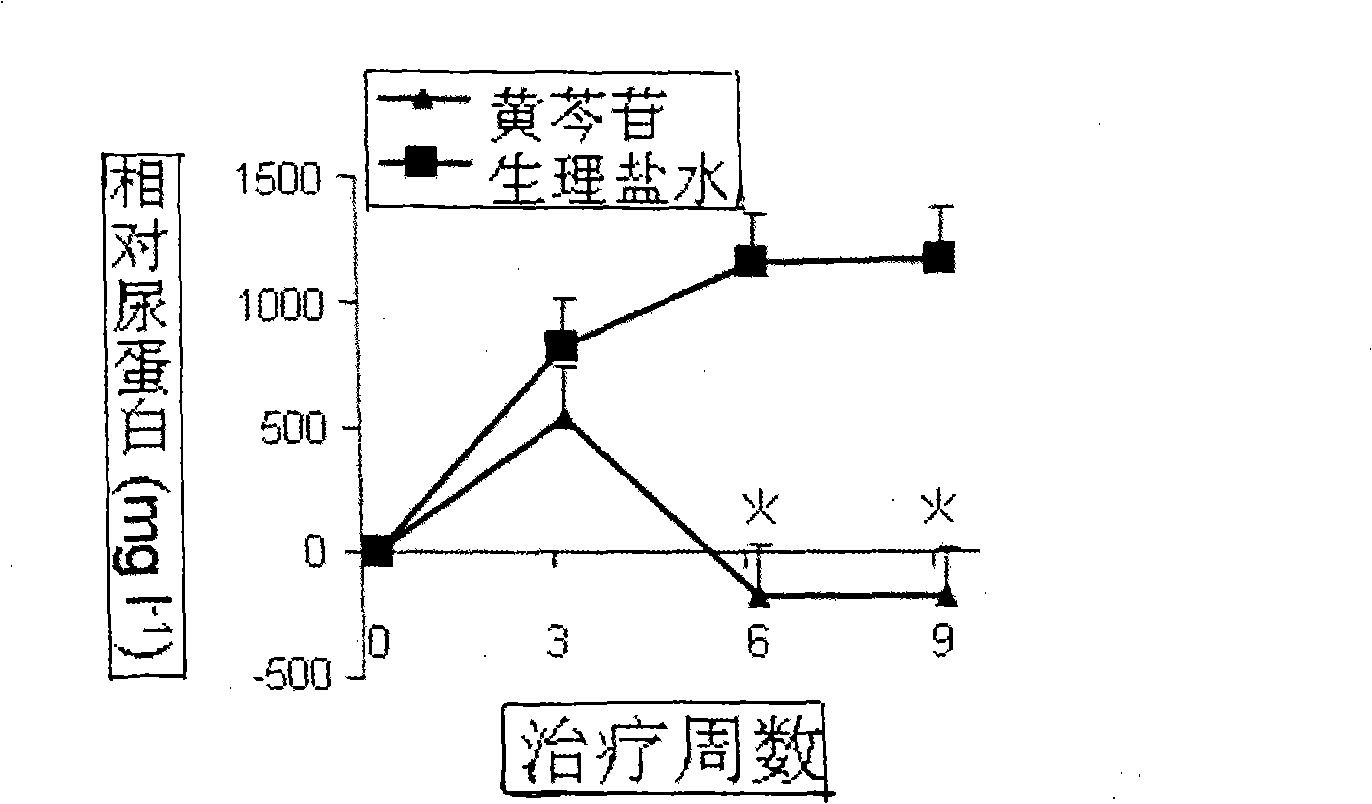
Use of baicalin in preparing medicine
InactiveCN101491533APromote conversionInhibit transformationOrganic active ingredientsAntipyreticVasculitisCurative effect
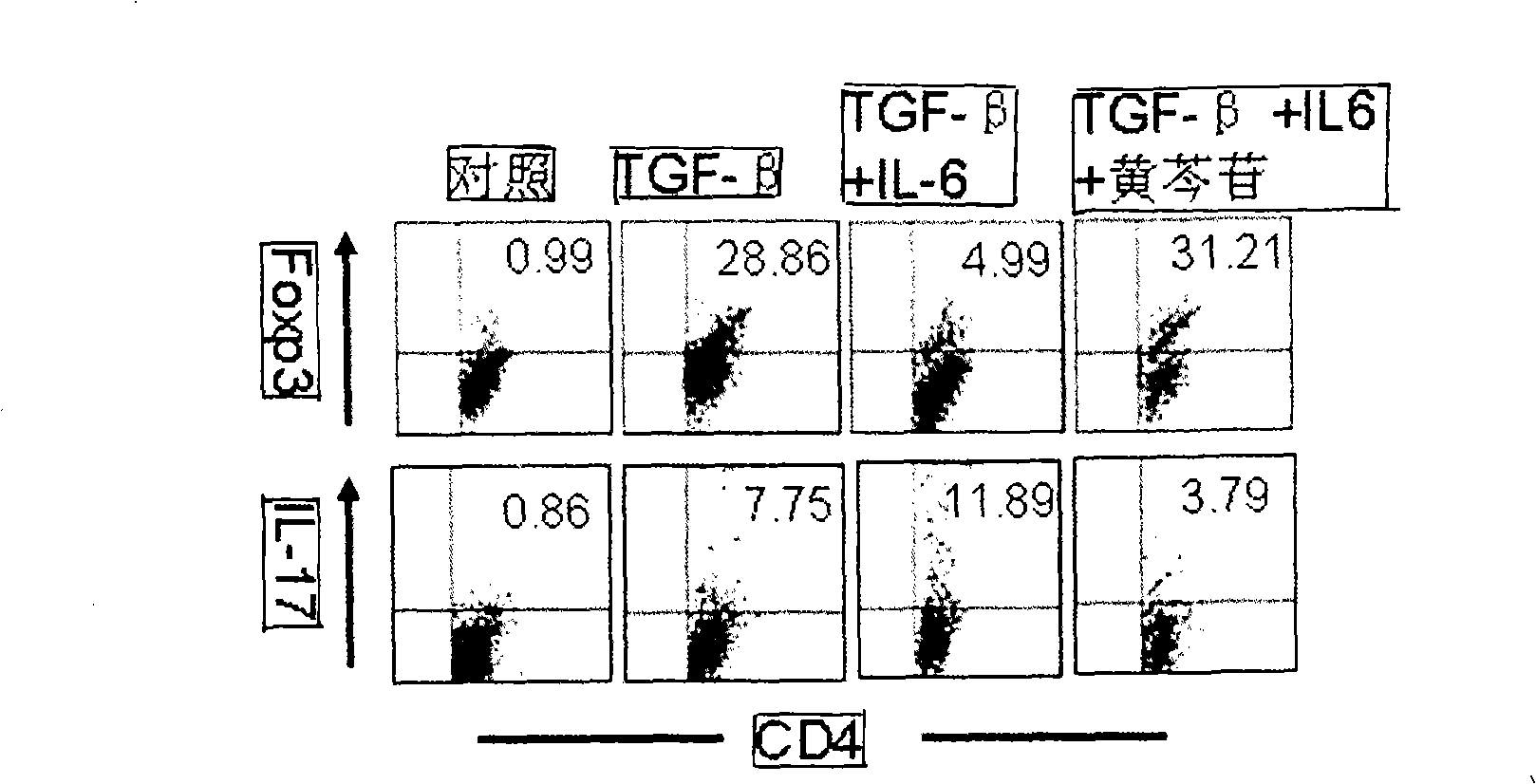
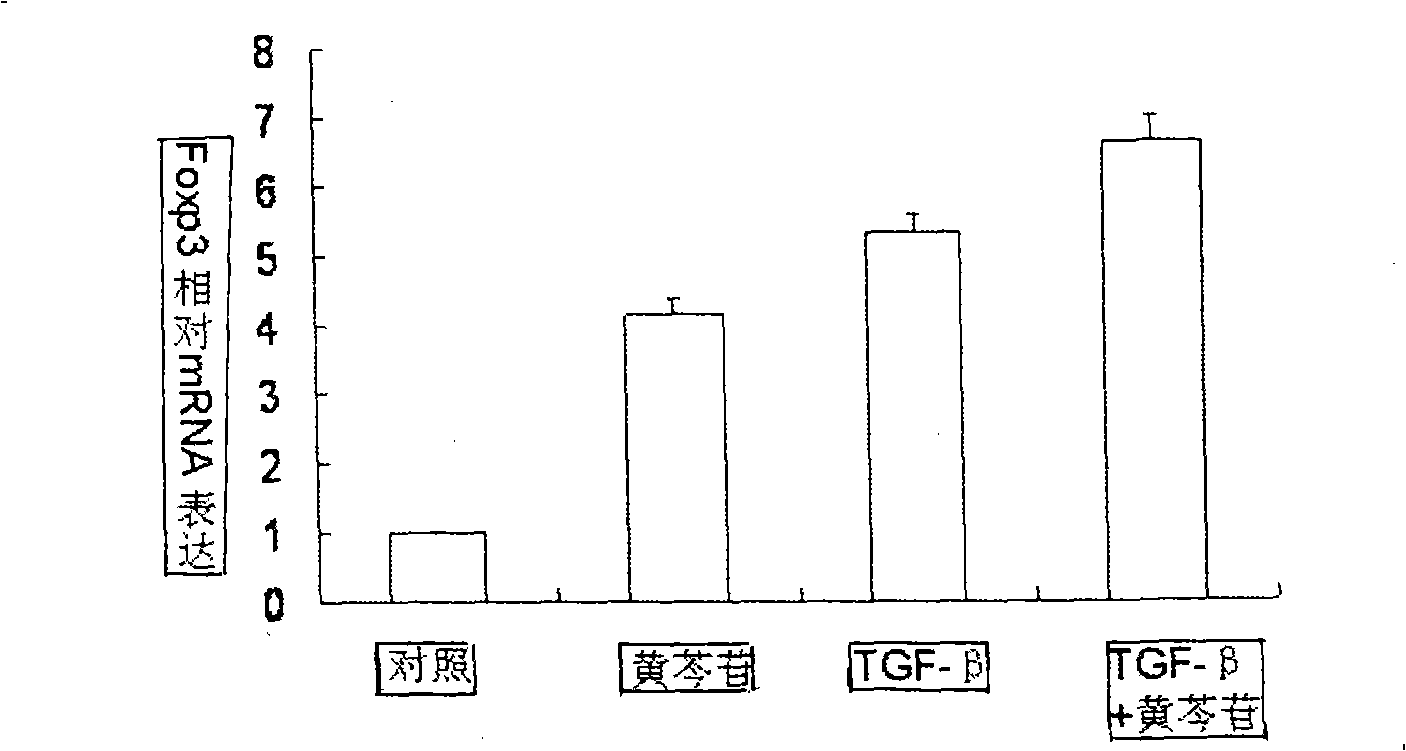
The invention relates to application of scutelloside in pharmacy. The scutelloside promotes a T cell to be transformed toward Treg with immunological suppression effect by up-regulating the expression of Foxp3; at the same time, the scutelloside inhibits Th17 with pro-inflammatory effect and inhibits vasculitis mediated by the Th17. A main mechanism of inhibting the Th17 of the scutelloside is to inhibit the amplification of the Th17 mediated by IL-6. At the same time, a further experiment proves that the scutelloside taken as a novel immunomodulator plays good therapeutic effect on treating lupus erythenlatosus nephritis and rheumatoid arthritis.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
Method and detection kit to judge whether solid tumors are suited for immunotherapy
ActiveCN108624650AIncreased objective response rateMicrobiological testing/measurementMaterial analysisPeripheral blood mononuclear cellCD16
The invention provides a detection kit to judge whether solid tumors are suited for immunotherapy. The detection kit includes detection reagents for detecting the indexes: tumor mutation load of peripheral blood circulating tumor DNA, HLA typing, HLA state of loss of heterozygosity, PD-L1 membrane expression positive tumor cell percentage, infiltration level of CD8+ immune cells, infiltration level of FOXP3+ immune cells, mRNA expression score of T-cell inflammation related genes, percentage of CD14+CD16-HLA-DR+ monocytes in peripheral blood mononuclear cells, and micro-satellite instability.The invention also provides, correspondingly, a method to judge whether solid tumors are suited for immunotherapy. The kit and method herein can be used to screen patients with solid tumors so as to provide significantly increased objective response rate and have a promising application prospect in terms of clinical screening of patients with solid tumors suited for immunotherapy.
Owner:LEPU MEDICAL TECH (BEIJING) CO LTD
Polygene transfection tumor cell strain and fusion vaccine thereof, as well as preparation methods
InactiveCN104099297AEnhanced anti-pancreatic cancer immune effectPresentation is validGenetic material ingredientsAntibody medical ingredientsDendritic cellT lymphocyte
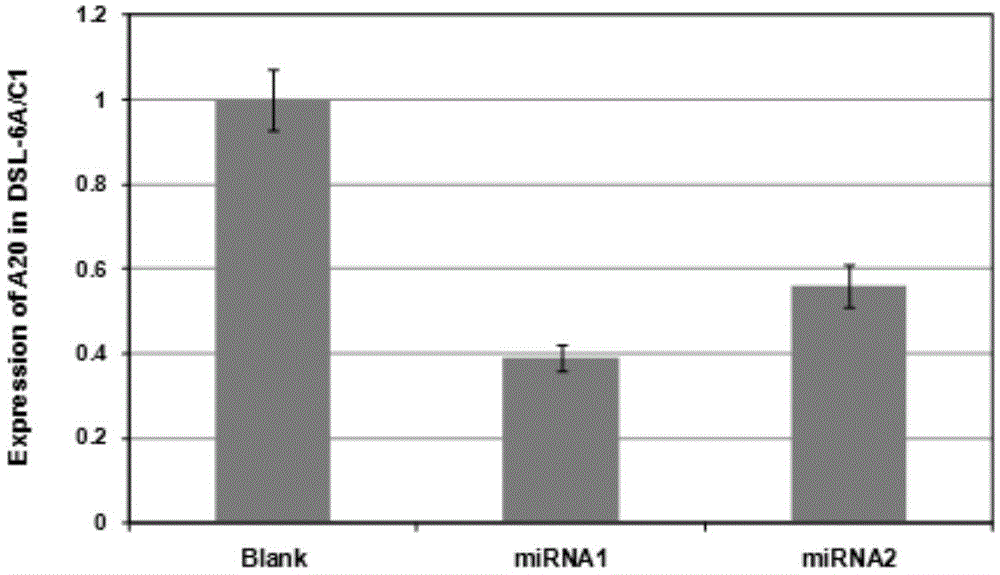
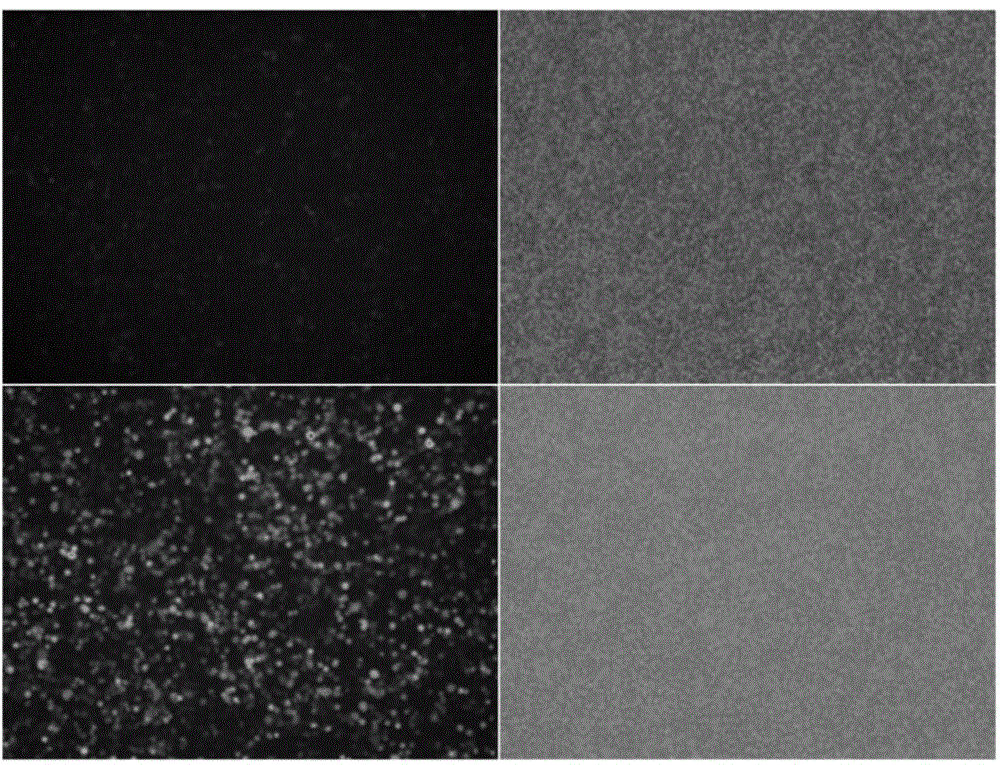
The invention discloses a polygene transfection tumor cell strain and a fusion vaccine thereof, as well as preparation methods. The cell strain comprises an Foxp3 gene and an A20 gene, and adopts the pancreatic cancer tumor cell line DSL6A / C1 of a rat as a transfection cell. The preparation methods of thepolygene transfection tumor cell strain and the fusion vaccine comprise the following steps: modifying the fusion vaccine of DSL6A / C1 cell and a dendritic cell of Treg by building over-expression Foxp3 and A20miRNA, so as to obtain a novel tumor vaccine which not only has a DC special function but also can express tumor antigen; adopting the fusion vaccine of the tumor cell and the dendritic cell which are modified through co-transfection of Foxp3-A20 to further enhance the anti-pancreatic cancer immunity effect. The fusion vaccine has not only a complete tumor antigen, but also the characteristic of an antigen presenting cell, and can effectively present an tumor antigen to a T lymphocyte, thereby stimulating an organism to generate a specific anti-tumor immunity response, being a new direction of tumor immunological therapy, providing a new method for curing tumor patients, and having a potential application value.
Owner:AFFILIATED HOSPITAL OF NANTONG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com