Heat treatment induces solubility change and degradation of pml/rarα fusion protein and its mutants
A fusion protein, soluble technology, applied in the medical field to achieve the effect of speeding up the treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
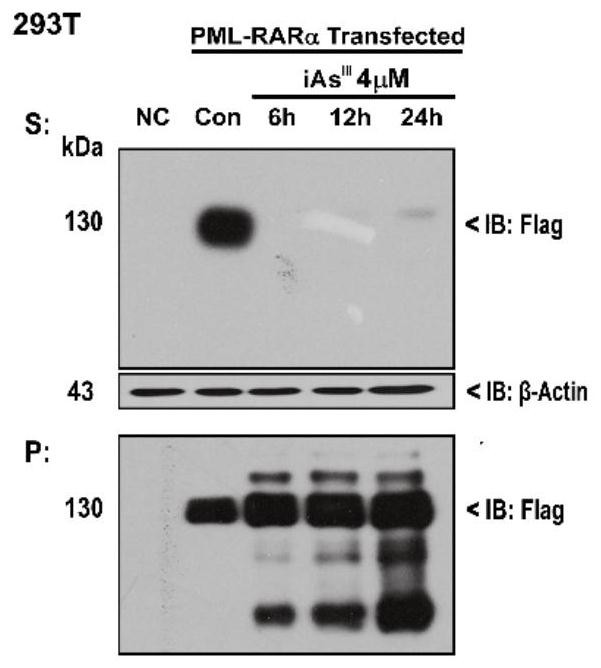
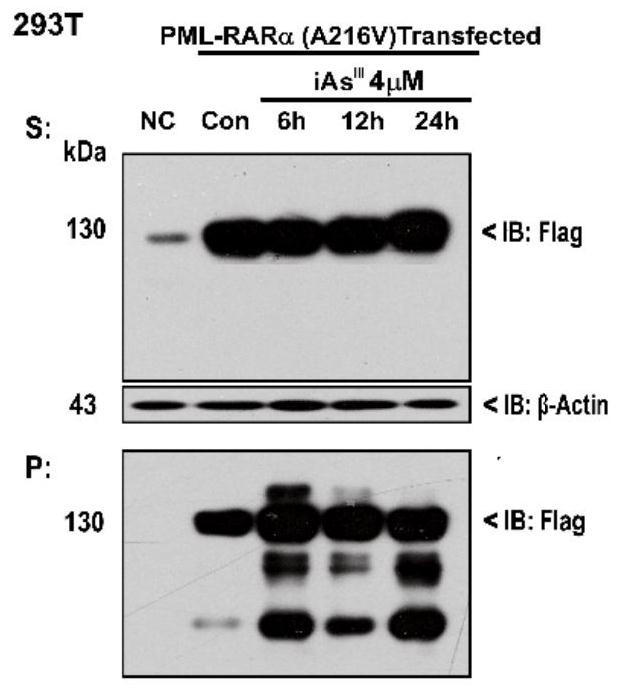
[0033] Example 1: As 2 o 3 (iAs III ) in vitro on the solubility transition of mutant A216V that induces PML / RARα and arsenic resistance
[0034] 293T cells were cultured in DMEM (purchased from Gibco) medium containing 10% fetal bovine serum (FBS) (37°C, 5% CO 2 , saturated humidity), after resuscitating, take the fourth generation cells and press (5~10)×10 4 / mL concentration to inoculate 5ml in T25 culture flask. Wait for the cells to grow to the logarithmic growth phase, and use Lipofectamine2000 (purchased from Thermo Fisher Scientific) to transfect PML / RARα ( figure 1 ) and its drug-resistant mutant A216V ( figure 2 ). Add As 24h after transfection 2 o 3 (4μM) for different time and collect the samples, blot the supernatant and store the cell samples at -80°C or directly lyse. When the cells are lysed, add a certain amount of RIPA lysate to the cell mass, and immediately pipette evenly. Place on ice, pipette several times every 10 min and vortex with a vortex ...
Embodiment 2
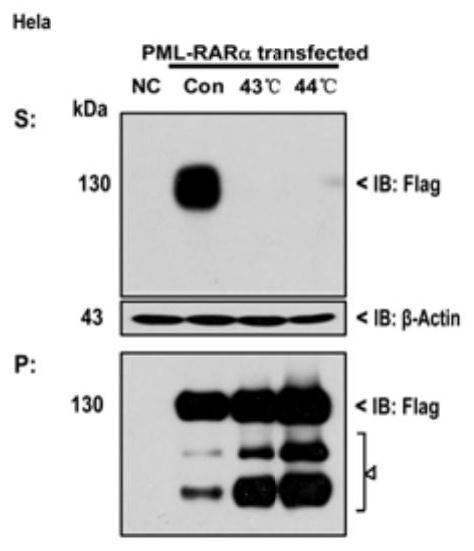
[0035] Example 2: Effect of Heat Shock on Inducing PML / RARα, PML (1-552) and RARα's Solubility Transformation in Vitro
[0036] Overexpress PML / RARα, PML(1-552) and RARα in HeLa cells using the method of Example 1, and heat shock treatment at 43°C for different times after 24h, such as Figure 3-5 shown. Collect samples, blot dry supernatant and store cell samples at -80°C or directly lyse. When the cells are lysed, add a certain amount of RIPA lysate to the cell mass, and immediately pipette evenly. Place on ice, pipette several times every 10 min and vortex with a vortex shaker. After 30 minutes, centrifuge at 13,000 rpm and 4°C for 30 minutes, take the supernatant, which is the supernatant protein solution (S), wash the precipitate with PBS 3 times, and dissolve it with LDS lysate to obtain the precipitated protein (P). The protein was quantified to a uniform concentration by the Lowrry method, and the protein solution was stored at -80°C or directly loaded on Western bl...
Embodiment 3
[0037] Example 3: As 2 o 3 (iAs III ), heat shock and their combined application on the solubility transition of PML / RARα fusion protein
[0038] Overexpression of PML / RARα, As in HeLa cells by the method of Example 1, 24h 2 o 3 , heat shock at 43°C and combined treatment of the two for different periods of time, such as Figure 6 ,7,8. Collect samples, blot dry supernatant and store cell samples at -80°C or directly lyse. When the cells are lysed, add a certain amount of RIPA lysate to the cell mass, and immediately pipette evenly. Place on ice, pipette several times every 10 min and vortex with a vortex shaker. After 30 minutes, centrifuge at 13,000 rpm and 4°C for 30 minutes, take the supernatant, which is the supernatant protein solution (S), wash the precipitate with PBS 3 times, and dissolve it with LDS lysate to obtain the precipitated protein (P). The protein was quantified to a uniform concentration by the Lowrry method, and the protein solution was stored at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com