COMT inhibitors with terminal olefinic bond and hydrazide structures as well as preparation method and use of COMT inhibitors
A technology of hydrazine hydrate and cyclopentadiene, which can be used in the fields of nervous system diseases, organic chemistry, drug combination, etc., and can solve problems such as limited applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
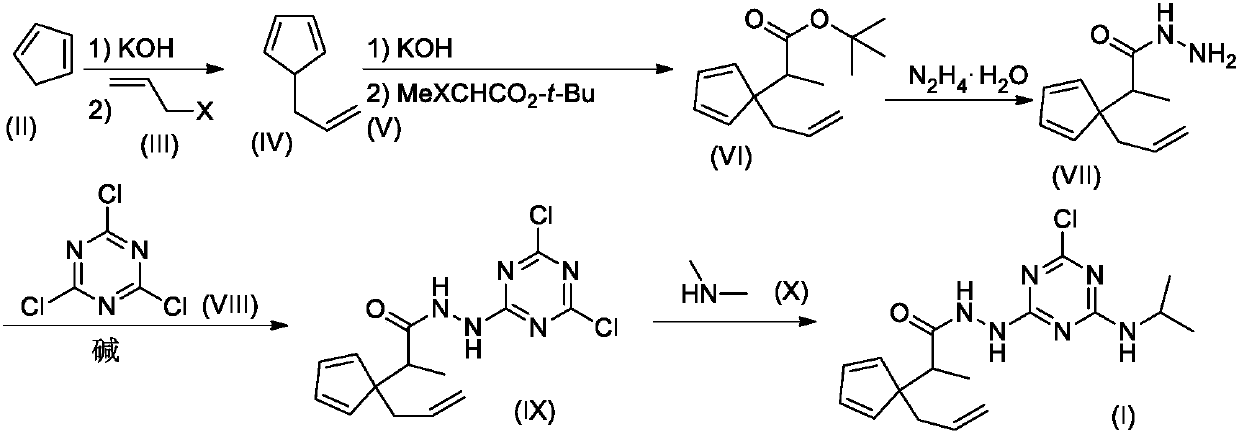
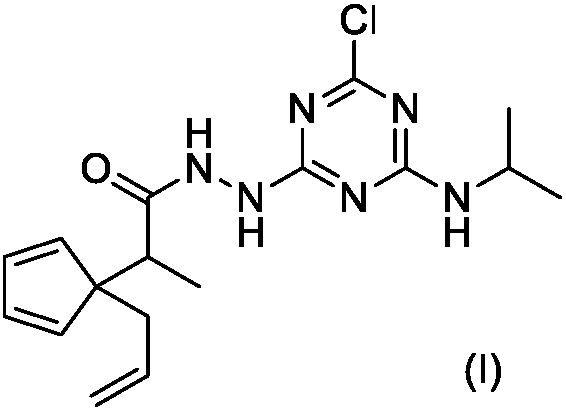
[0020] The synthesis of embodiment 1 compound I-1
[0021]
[0022] Step 1. Synthesis of Compound VI-1
[0023] Compound II (1.32g, 20mmol) was dissolved in 20mL DMSO, stirred at room temperature, KOH solid (2.24g, 40mmol) was added, and stirred at room temperature for 1 hour. Further MeI (III-1, 2.84 g, 20 mmol) was added, and stirring was continued overnight at room temperature. Then tert-butyl bromoacetate V (3.90 g, 20 mmol) was added, and the stirring was continued for 12 hours. TLC detection found that the reaction was complete. The reaction mixture was carefully poured into 200mL ice water, stirred, and washed with 50mL×3CH 2 Cl 2 After extraction, the extract phases were combined, washed with 100 mL of 5% brine, and dried over anhydrous sodium sulfate. The desiccant was removed by suction filtration, the filtrate was evaporated to dryness on a rotary evaporator, and the residue was purified by silica gel column chromatography to obtain compound VI-I, 2.99 g (co...
Embodiment 2
[0028] The synthesis of embodiment 2 compound 1-2
[0029]
[0030] Step 1. Synthesis of compound VI-2
[0031] Compound II (1.32g, 20mmol) was dissolved in 20mL DMSO, stirred at room temperature, KOH solid (2.24g, 40mmol) was added, and stirred at room temperature for 1 hour. III-2 (2.42 g, 20 mmol) was added again, and stirring was continued overnight at room temperature. Then add V 20mmol again, continue stirring for 12 hours, TLC detection finds that the reaction is complete. The reaction mixture was carefully poured into 200mL ice water, stirred, and washed with 50mL×3CH 2 Cl 2After extraction, the extract phases were combined, washed with 100 mL of 5% brine, and dried over anhydrous sodium sulfate. The desiccant was removed by suction filtration, the filtrate was evaporated to dryness on a rotary evaporator, and the residue was purified by silica gel column chromatography to obtain compound VI-2. ESI-MS, m / z=235 ([M+H] + ).
[0032] Step 2. Synthesis of Compo...
Embodiment 3
[0036] Example 3 Compound Inhibition COMT Analysis in Vitro
[0037] The COMT inhibitory activity of the compounds of the present invention was determined using the experimental method described below. The fluorescence assay is based on the methylation of a substrate (6,7-dihydroxycoumarin) by COMT to generate a highly fluorescent product (7-hydroxy-6-methoxycoumarin). The reaction requires the presence of magnesium ions and a methyl donor, in this case S-adenosylmethionine (SAM)]. A 10-point 3-fold dilution series was prepared using 10 mM compound stocks in DMSO and 1 μL of the appropriate dilution was placed in assay wells (black 96-well round bottom polystyrene plates from Costar; cat. no. 3792). Dilute the recombinant enzyme in assay buffer (100mM Na 2 HPO 4 pH 7.4, 1 mM DTT, 0.005% Tween-20) and 35 μL was added to assay wells containing 1 μL of compound. Pre-incubation of COMT enzyme and compound was performed for 2 hours at room temperature. Use 5 μL containing 40...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com