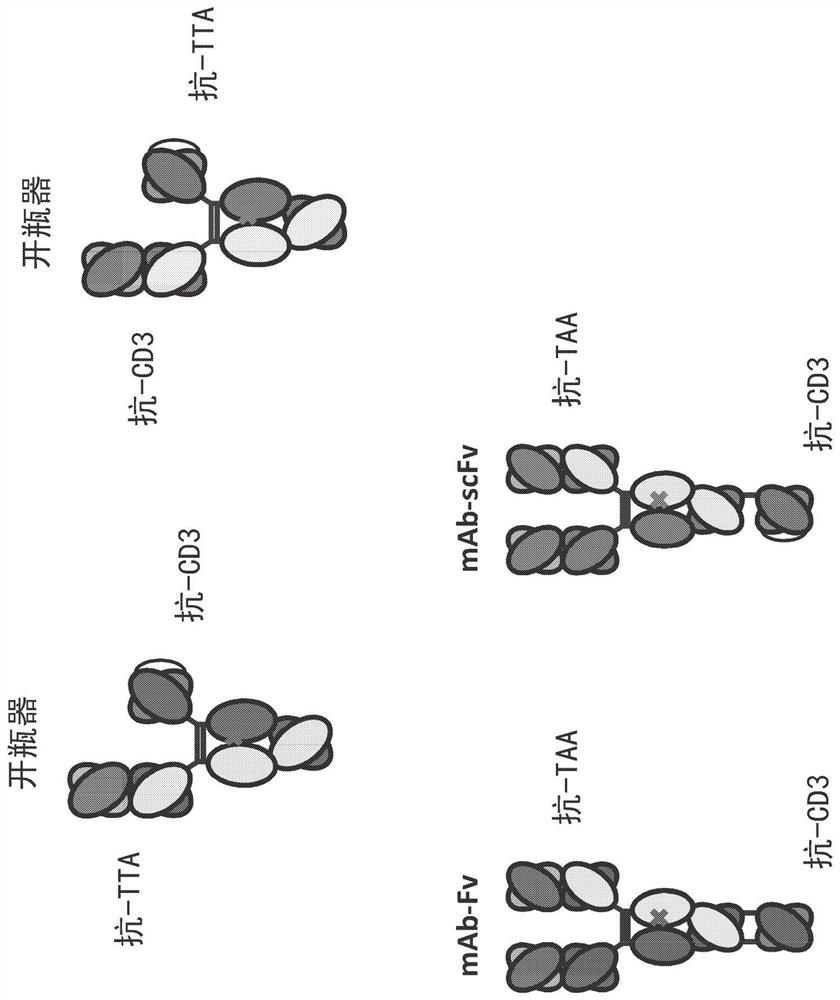
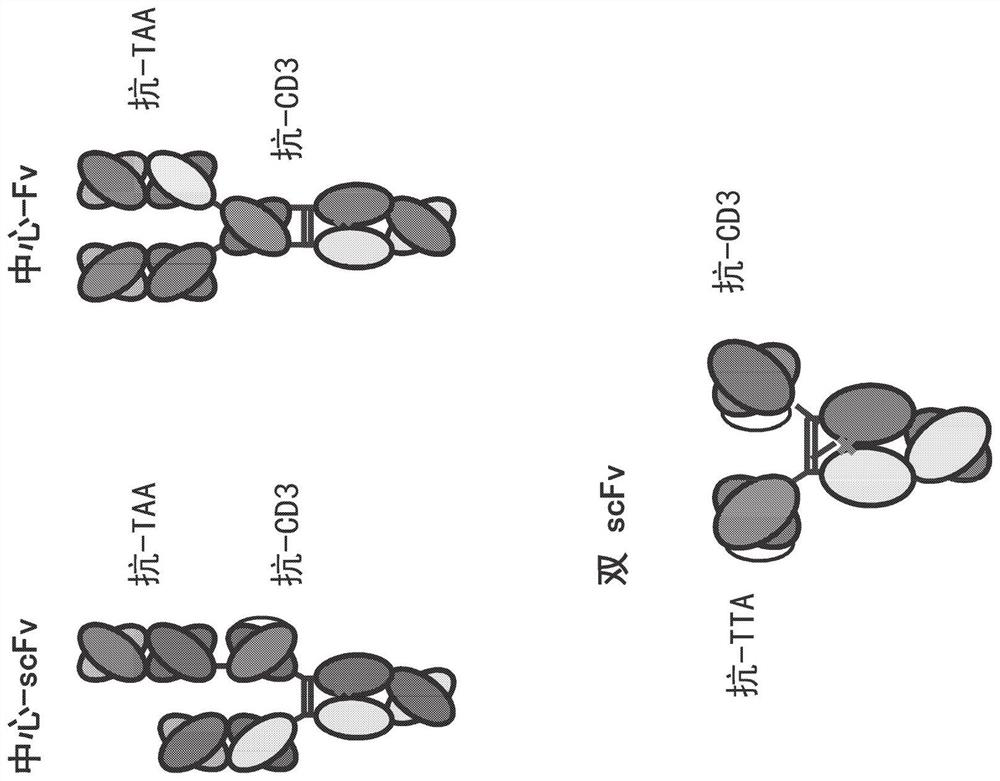
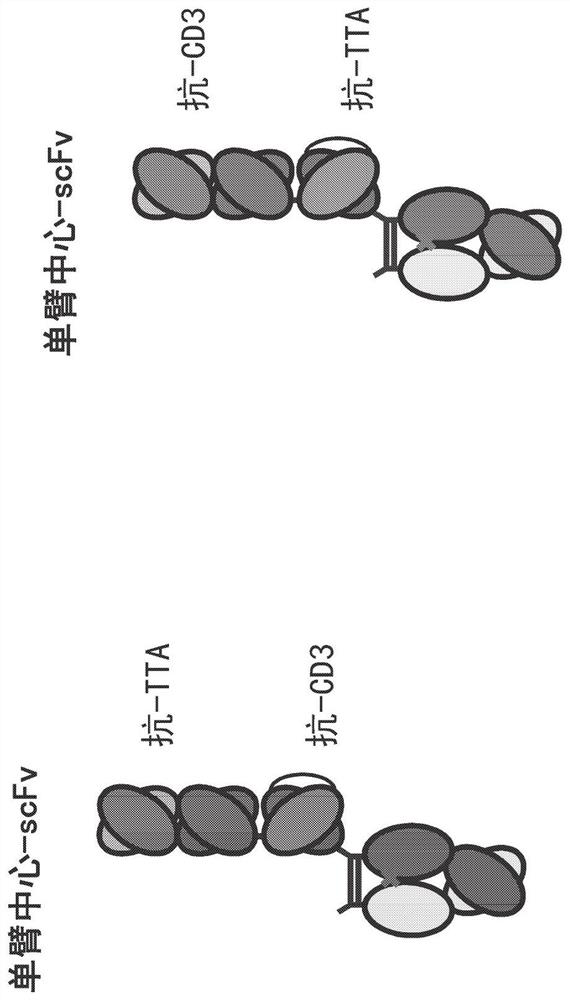
Heterodimeric antibodies that bind CD3 and tumor antigens
A heterodimer and antibody technology, applied in the direction of antibodies, anti-tumor drugs, antibody medical components, etc., can solve the problems of limited biophysics and pharmacokinetics, toxic effects, non-specific activation of bispecific antibodies, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0456] Embodiment 1: Transformation form
[0457] Production of bispecific antibodies
[0458] The schematic diagram of anti-CD38×anti-CD3 bispecific antibody is shown in FIG. 1 . The amino acid sequences of various transformed anti-CD38×anti-CD3 bispecific antibodies are listed in Figure 39 to Figure 43 middle. DNA encoding the three strands required for bispecific antibody expression was generated by gene synthesis (Blue Heron Biotechnology, Bothell, WA) and subcloned into the expression vector pTT5 using standard molecular biology techniques. Substitutions were introduced using site-directed mutagenesis (QuikChange, Stratagene, Cedar Creek, TX) or other gene synthesis and subcloning. DNA was transfected into HEK293E cells for expression, and the resulting protein was purified from the supernatant using protein A affinity chromatography (GE Healthcare) and cation exchange chromatography. The yield of protein A affinity purification is as follows Figure 35 shown. Cati...
Embodiment 2
[0462] Redirected T cell cytotoxicity
[0463] In vitro identification of redirected T cell cytotoxicity (RTCC) of an anti-CD38×anti-CD3 Fab-scFv-Fc bispecific antibody against the CD38+ RPMI8266 myeloma cell line. 40k RPMI8266 cells were incubated with 400k human PBMCs for 96 hours. Measurement of RTCC by flow cytometry, as Figure 44 shown. CD69, Ki-67 and PI-9 expression of CD4+ and CD8+ T cells were also identified by flow cytometry, as Figure 45 shown.
[0464] Mouse Model of Anti-Tumor Activity
[0465] On the -23rd day, five NODscidγ (NSG) mice in each group were implanted into the tail vein of four groups with 5 × 10 6 RPMI8226TrS tumor cells (multiple myeloma, expressing luciferase). On day 0, mice were intraperitoneally implanted with 10×10 6 Individual PBMCs. After implantation of PBMCs on day 0, test articles were administered weekly (days 0 and 7) by intraperitoneal injection at dose levels as follows: Figure 4 shown. Figure 46 The experimental design...
Embodiment 3
[0471] CDR development of CD123
[0472] The starting point for developing the CDRs of the humanized CD123 antibody Fab is the variable and light chain regions of the 7G3 murine antibody, called "7G3H0L0", from ATCC HB-12009. However, the initial humanization (H1_L1, see Figure 136 for the sequence) resulted in a severe reduction in affinity (5 to 10 fold affinity, see Figure 156B and C ). This decrease in affinity was mainly due to humanization of the heavy chain, see H1_L0 constructs (eg first humanized heavy chain with murine light chain), the H1_L1 construct showed a full 10-fold decrease. Consistent with this was a 10-fold reduction in RTCC (redirected T cell cytotoxicity) potency, see Figure 156D , the test is aimed at KG1a cells expressing CD123.
[0473] Thus, two rounds of affinity / stabilization optimization were performed. Round 1 ("Library 1" see Figure 157 ) generated 108 variants including LDA, targeting and back substitutions, these were then affinity ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com