A method for co-producing 3,3,3-trifluoropropylene carbonate and 3,3,3-trifluoro-1,2-propanediol
A technology of trifluoropropylene carbonate and propylene glycol, applied in hydrolysis preparation, chemical instruments and methods, preparation of hydroxyl compounds, etc., can solve the problems of complex reaction system, harsh reaction conditions, low reaction selectivity, etc., and achieve high reaction yield , mild reaction conditions, good atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
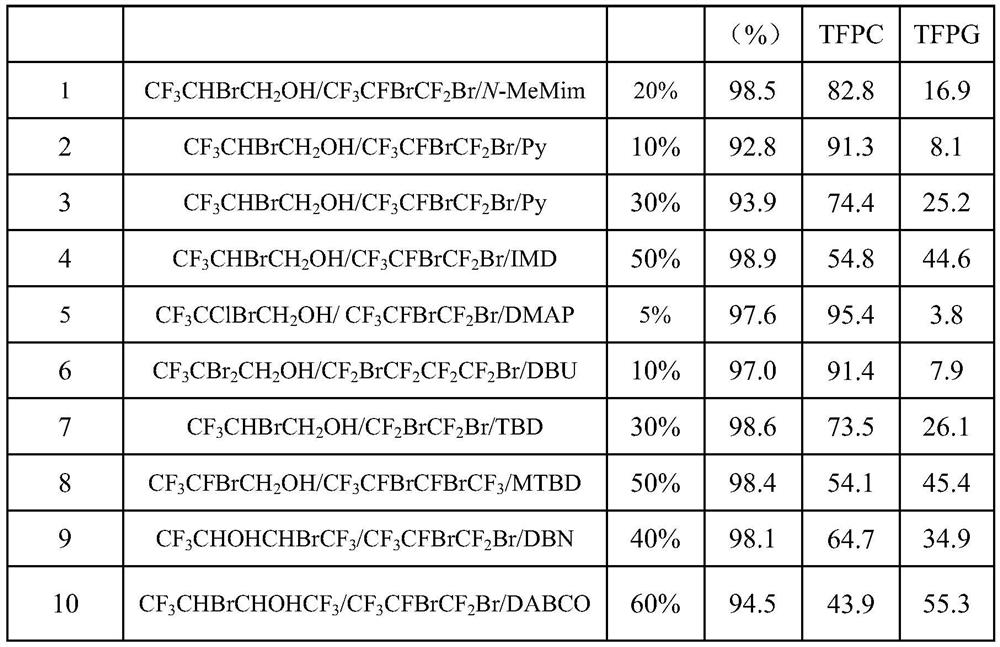
[0028] 3,3,3-trifluoropropylene oxide (33.6g, 0.3mol) and 2-bromo-1,1,1-trifluoro-3- Propanol (1.5g, 7.5mmol), 2,3-dibromo-1,1,1,2,3,3-hexafluoropropane (4.6g, 15mmol), N-methylimidazole (1.2g, 15mmol) , after sealing with CO 2 Replace the reactor twice, start stirring and heat to 100°C, and continuously feed CO 2 Keep the reaction pressure at 0.5MPa, cool to room temperature after 4 hours of reaction, and slowly release excess CO 2 gas, then add 20% water of 3,3,3-trifluoropropylene oxide molar weight to the reaction solution, and stir the reaction for about 12 hours at 60°C to obtain 3,3,3-trifluoropropylene carbonate and The crude product of 3,3,3-trifluoro-1,2-propanediol was analyzed by gas chromatography, and the target product 3,3,3-trifluoropropene carbonate and 3,3, A total of 44.2 g of 3-trifluoro-1,2-propanediol was calculated and the yield was calculated. The reaction results are shown in Table 1.
[0029] Gas chromatography analysis conditions: Agilent 7820 ga...
Embodiment 2
[0031] In a 500mL stainless steel autoclave equipped with mechanical stirring, 3,3,3-trifluoropropylene oxide (336g, 3mol) and 2-bromo-1,1,1-trifluoro-3-propane were sequentially added with a moisture content of 3000ppm Alcohol (28.9g, 0.15mol), 2,3-dibromo-1,1,1,2,3,3-hexafluoropropane (92.94g, 0.3mol), pyridine (23.7g, 0.3mol), after sealing Using CO 2 Replace the reactor twice, start stirring, set the reaction temperature to 25°C, and continuously feed CO 2 Keep the reaction pressure at 5MPa, and slowly release excess CO after 48 hours of reaction 2 gas, then add 10% water of 3,3,3-trifluoropropylene oxide molar weight to the reaction solution, and stir the reaction for about 24 hours at 40°C to obtain 3,3,3-trifluoropropylene carbonate and The crude product of 3,3,3-trifluoro-1,2-propanediol was analyzed by gas chromatography for reaction product distribution, and the target product 3,3,3-trifluoropropene carbonate and 3,3,3 -A total of 425.7g of trifluoro-1,2-propanedi...
Embodiment 3
[0033] In a 500mL stainless steel autoclave equipped with mechanical stirring, 3,3,3-trifluoropropylene oxide (336g, 3mol) and 2-bromo-1,1,1-trifluoro-3-propane were sequentially added with a moisture content of 5000ppm Alcohol (5.8g, 0.03mol), 2,3-dibromo-1,1,1,2,3,3-hexafluoropropane (9.3g, 0.03mol), pyridine (2.4g, 0.03mol), after sealing Using CO 2 Replace the reactor twice, start stirring, set the reaction temperature to 150°C, and continuously feed CO 2 Keep the reaction pressure at 0.1MPa, cool to room temperature after 2 hours of reaction, and slowly release excess CO 2 gas, then add 30% water of 3,3,3-trifluoropropylene oxide molar weight to the reaction solution, and then stir the reaction at 30°C for about 48h to obtain 3,3,3-trifluoropropylene carbonate With 3,3,3-trifluoro-1,2-propanediol crude product, the reaction product distribution analysis is carried out by gas chromatography, and the target product 3,3,3-trifluoropropene carbonate and 3,3, A total of 415...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap