Preparation and application of indole derivative
A technology of indole derivatives and methanol, applied in the field of microbial herbicides, can solve problems such as endangering human health, environmental pollution, weed resistance, and endangering the safety of agricultural products, and achieve low product cost, good herbicidal activity, and short cycle Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
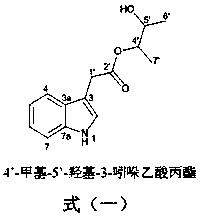
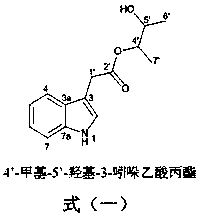
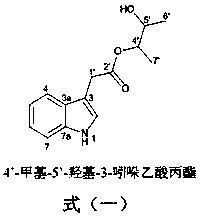
[0022] Example 1: Indole derivatives of chlamydosporin type are shown in formula (1) (the Arabic numerals in the structure are the standard positions of carbon atoms).
[0023]
Example Embodiment
[0024] Example 2: Fermentation production, separation and purification of chlamydosporin indole derivatives:
[0025] 1) Fermentation culture
[0026] Strain culture: According to the conventional culture method of microorganisms, select a small amount of strains stored in agar-malt extract medium Fusarium chlamydosporum QJP1, inoculated on the surface of the PDA plate, cultured at 28°C for 3 days, as a strain for large-scale fermentation culture, ready for use.
[0027] Cut an appropriate amount of bacteria on the surface of the PDA plate, inoculate it into a sterilized Erlenmeyer flask containing rice culture medium, and let it stand for 30 days at room temperature. Sterilize by adding ethyl acetate and set aside.
[0028] The rice culture medium is 100 g / bottle of rice, 0.6 g / bottle of peptone, and 100 mL / bottle of artificial sea water.
[0029] 2) Separation and purification of compounds
[0030] The above-mentioned rice culture medium was ultrasonically extracted 3 times with eth...
Example Embodiment
[0033] Example 3: Herbicidal activity test
[0034] The agar powder mixing method established by Luo Xiaoyong et al. [Journal of Qingdao Agricultural University, 2007; 24(4): 267-270] is used to quickly determine the herbicidal activity of compounds, with high sensitivity, simple operation, short determination time, and good repeatability. , The characteristics of large-throughput and extensive screening, and easy to observe the growth status of indicating plants. Luo Xiaoyong et al. used this method to quickly determine the herbicidal activity of 40 garden plant leaves. Zhang Lijuan [Plant Protection, 2016; 42:63-66.] and Su Fansheng [Applied Chemistry, 2014;31:290-295.] Using barnyardgrass as the test weed, the corn stalk was quickly determined by the agar mixture method And the herbicidal activity of imidazole compounds. Zhang Yun[Journal of Microbiology, 2015;55(3):292-298.] and Shuai Li[Journal of Agricultural and Food Chemistry, 2014; 62(14):8997-9001.] Taking amaranthus ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com