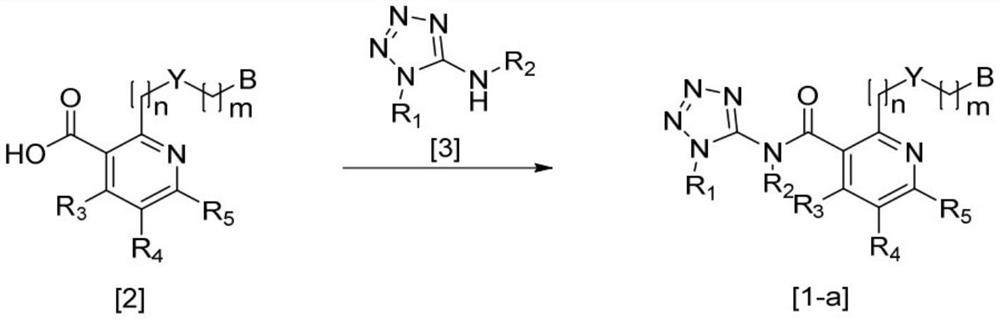
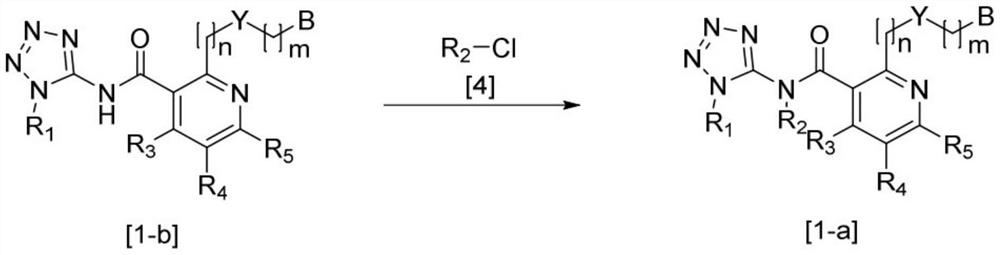
Nicotinamide compounds and herbicide compositions comprising same
A compound, nicotinamide technology, used in herbicides and algicides, biocides, animal repellants, etc., can solve problems such as inability to solve the problem of drug resistance, and achieve excellent crop selectivity, excellent herbicidal activity, excellent effect of removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
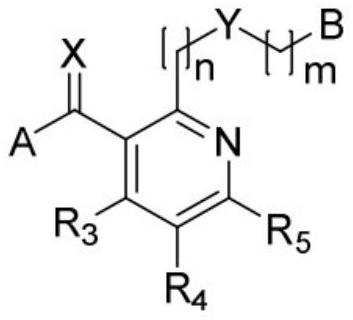
[0036] Embodiment 1. The present invention provides a compound selected from the group consisting of nicotinamide compounds represented by the following Chemical Formula 1 and agrochemically acceptable salts thereof.
[0037] [Chemical formula 1]
[0038]
[0039] In Chemical Formula 1,
[0040] A is
[0041] B is
[0042] X is O or S;
[0043] Y is O, S, SO, SO 2 , NH, N-(C 1 -C 6 ) alkyl, N-O-(C 1 -C 6 ) alkyl, N-S-(C 1 -C 6 ) alkyl, N-S(O)-(C 1 -C 6 ) alkyl, N-SO 2 -(C 1 -C 6 ) alkyl, N-O-(C 1 -C 3 ) alkyl-aryl or hydroxyamino;
[0044] Z is N or C-R 8 ;
[0045] R 1 for (C 1 -C 6 ) alkyl, (C 1 -C 6 ) haloalkyl, (C 2 -C 6 ) alkenyl, (C 2 -C 6 ) haloalkenyl, (C 2 -C 6 ) alkynyl, (C 2 -C 6 ) haloalkynyl, (C 3 -C 6 ) cycloalkyl or (C 3 -C 6 ) halocycloalkyl;
[0046] R 2 for hydrogen, (C 1 -C 6 ) alkyl, (C 1 -C 6 ) haloalkyl, (C 2 -C 6 ) alkenyl, (C 2 -C 6 ) haloalkenyl, (C 2 -C 6 ) alkynyl, (C 2 -C 6 ) haloalkynyl, C(O)...
Embodiment approach 2
[0054] Embodiment 2. In the compound represented by Chemical Formula 1,
[0055] A is
[0056] B is
[0057] X is O or S;
[0058] Y is O, S, SO, SO 2 , NH, N-(C 1 -C 3 ) alkyl, N-O-(C 1 -C 3 ) alkyl, N-S-(C 1 -C 6 ) alkyl, N-S(O)-(C 1 -C 6 ) alkyl, N-SO 2 -(C 1 -C 6 ) alkyl, N-O-(C 1 -C 3 ) alkyl-aryl or hydroxyamino;
[0059] Z is N or C-R 8 ;
[0060] R 1 for (C 1 -C6 ) alkyl, (C 1 -C 6 ) haloalkyl, (C 2 -C 6 ) alkenyl or (C 2 -C 6 ) alkynyl;
[0061] R 2 for hydrogen, (C 1 -C 3 ) alkyl, (C 1 -C 3 ) haloalkyl, C(O)-(C 1 -C 6 ) alkyl, C(O) aryl or SO 2 -(C 1 -C 3 )alkyl;
[0062] R 3 or R 4 each independently hydrogen, halogen, (C 1 -C 6 ) alkyl or (C 2 -C 6 ) alkenyl;
[0063] R 5 is halogen or (C 1 -C 3 ) haloalkyl;
[0064] R 6 for (C 1 -C 6 ) alkyl, (C 1 -C 6 ) haloalkyl or (C 1 -C 3 ) alkyl-O-(C 1 -C 3 )alkyl;
[0065] R 7 for (C 1 -C 6 ) alkyl or (C 3 -C 6 ) cycloalkyl;
[0066] R 8 for hydrogen, (C ...
Embodiment approach 3
[0070] Embodiment 3. In the compound represented by Chemical Formula 1 according to any one of the preceding embodiments, A is
[0071] B is
[0072] X is O or S;
[0073] Y is O, S, SO, SO 2 , NH, methylamino, ethylamino, propargylamino, benzylhydroxyamino, methoxyamino, ethoxyamino or hydroxyamino;
[0074] Z is N or C-R 8 ;
[0075] R 1 is methyl, ethyl, propyl, difluoromethyl, allyl or propargyl;
[0076] R 2 is hydrogen, methyl, acetyl, benzoyl or methanesulfonyl;
[0077] R 3 or R 4 each independently hydrogen, chlorine, bromine, iodine, methyl, ethyl or vinyl;
[0078] R 5 is chlorine, difluoromethyl, trifluoromethyl, chlorodifluoromethyl or perfluoroethyl;
[0079] R 6 is methyl, ethyl, difluoromethyl or methoxymethyl;
[0080] R 7 is methyl, ethyl, isopropyl or cyclohexyl;
[0081] R 8 is hydrogen, methyl, fluoromethyl, difluoromethyl, trifluoromethyl, vinyl, methylvinyl, methoxy, trifluoroethoxy, methylthio, methanesulfonyl, dimethylamino , cyan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com