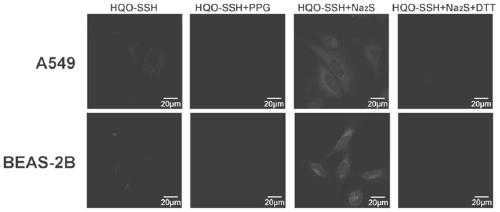
A fluorescent probe for detecting protein sulfhydrylation and its preparation method and application
A technique of fluorescent probes and groups, which is applied in the field of fluorescent probes for detecting protein thiolation and their preparation, can solve the problems of limited sensitivity, high false positive rate, complicated operation steps, etc., and achieves fast reaction speed and stability. Good, high sensitivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Synthesis of Compound I-2
[0038]
[0039] Dissolve IR780 (1g, 1.5mmol) and 2g sodium acetate in 20ml DMF and react at 80°C for 6 hours. The reaction solution is extracted with ethyl acetate and washed three times with water. Methane / methanol = 20:1 was used as eluent to pass through the column to obtain 600 mg of red solid I-2 with metallic luster, and the yield was 60%. 1 H NMR (400MHz, CDCl 3 )δ8.17(d, J=13.2Hz, 2H), 7.17(dd, J=7.0,5.6Hz, 4H), 6.90(t, J=7.4Hz, 2H), 6.67(d, J=8.0Hz, 2H), 5.46(d, J=13.2Hz, 2H), 3.64(t, J=7.4Hz, 4H), 2.61(t, J=5.7Hz, 4H), 1.88(dd, J=11.9, 6.1Hz, 2H), 1.76(dd, J=14.7, 7.4Hz, 4H), 1.67(s, 12H), 1.00(t, J=7.4Hz, 6H); 13 CNMR (100MHz, CDCl 3 )δ186.4, 162.4, 144.4, 139.7, 132.9, 127.6, 126.5, 121.7, 120.4, 106.7, 92.5, 77.4, 77.0, 76.7, 46.5, 44.1, 28.8, 25.9, 22.6, 19.8, 11.7; ESI-HRMS (m / z ):[M+H] + calculated for HQO(C 36 h 45 N 2 O, M + ):521.3532, found 521.3388.
[0040] Preparation of fluorescent probe I-1
[0041] ...
Embodiment 2
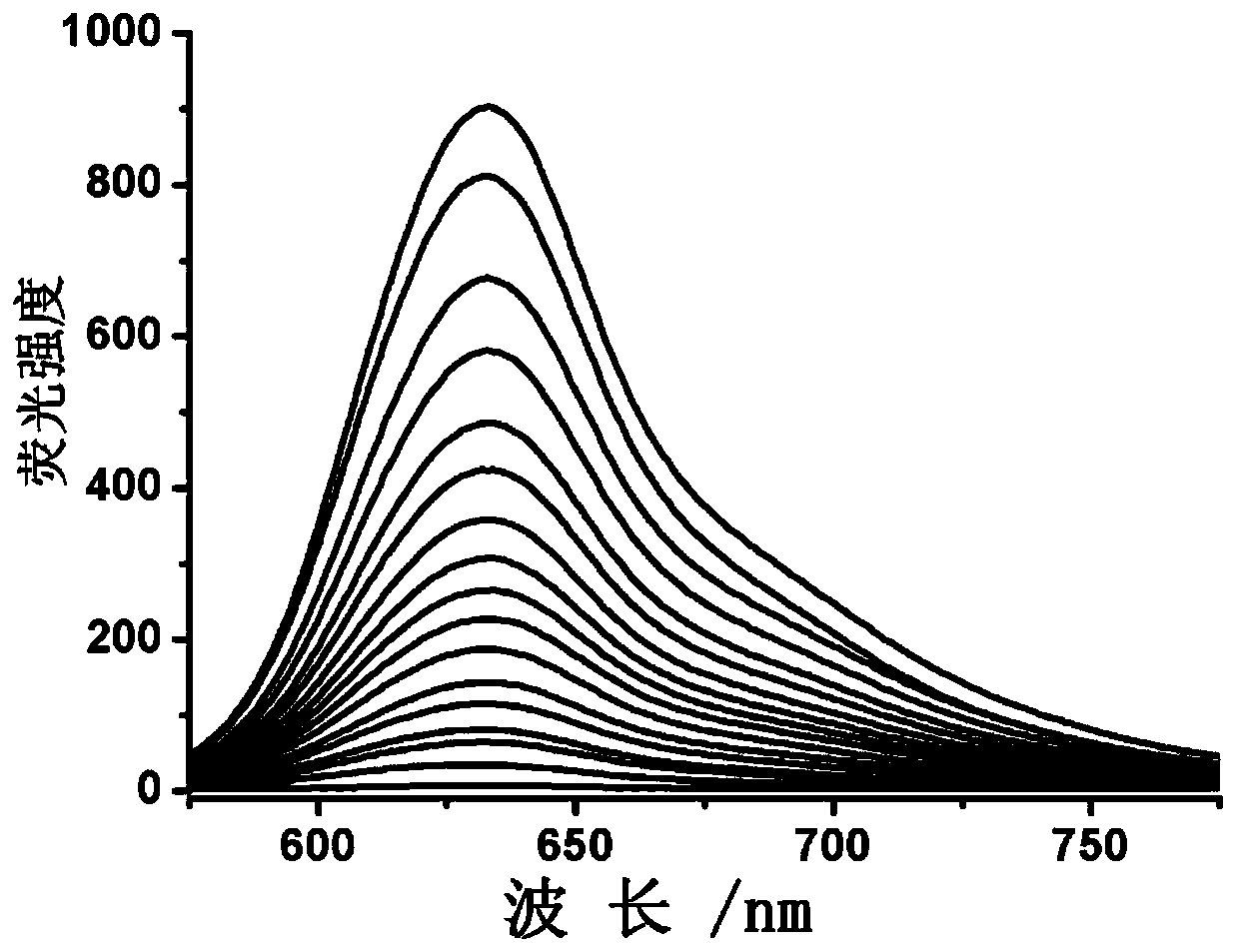
[0043] Embodiment 2, Fluorescence before and after the reaction of fluorescent 1 probe I-1 with different concentrations of sulfur-thiolated papain Variety
[0044] A small amount of fluorescent probe was dissolved in DMSO, and different concentrations of sulfur-sulfhydryl-modified papain were added. The final concentration of the probe was 20 μM. After reacting for 10 minutes, use 520nm excitation to record the solution at the maximum emission wavelength (640nm). Fluorescence intensity, record its fluorescence spectrum, such as figure 1 shown by figure 1 It can be seen that the fluorescent probe I-1 can react with the sulfur-thiolated protein and emit fluorescence.
Embodiment 3
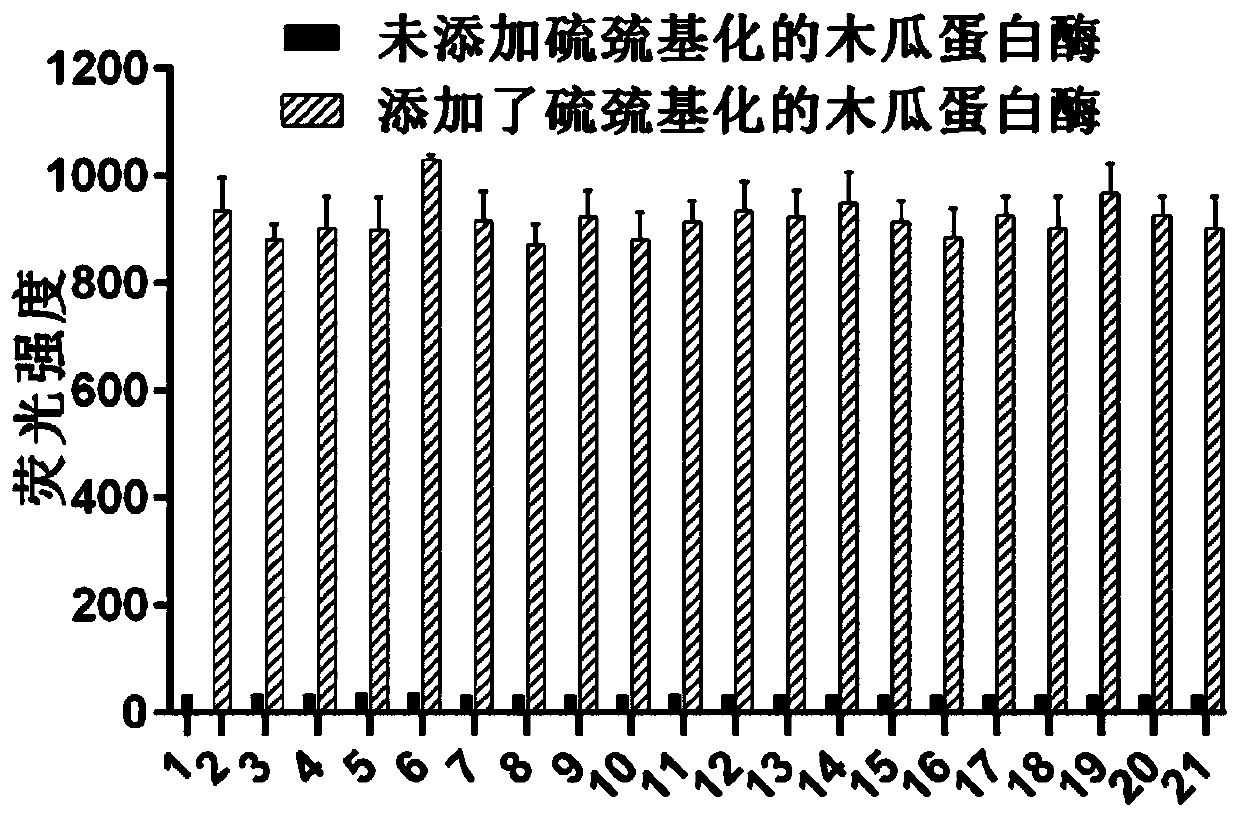
[0045] Embodiment 3, the selectivity of fluorescent probe I-1 to sulfur-thiolated protein
[0046] The fluorescent probes dissolved in DMSO were added to different solutions containing or not containing sulfhydryl-modified papain, from 1 to 21 were only blank group, only sulfhydryl-modified papain, SCN - , SO 3 2- , S 2 o 4 2- , S 2 o 5 2- , S 2 o 3 2- , S-nitroso glutathione, O 2 - 、H 2 o 2 , OCl - , tert-BuOOH, Ala, Arg, His, Me, Ser, Thr, Trp, Tyr, Val. Record the fluorescence intensity of their solutions respectively, such as figure 2 shown by figure 2 It can be seen that the fluorescent probe I-1 is selective for sulfhydrylated proteins.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com