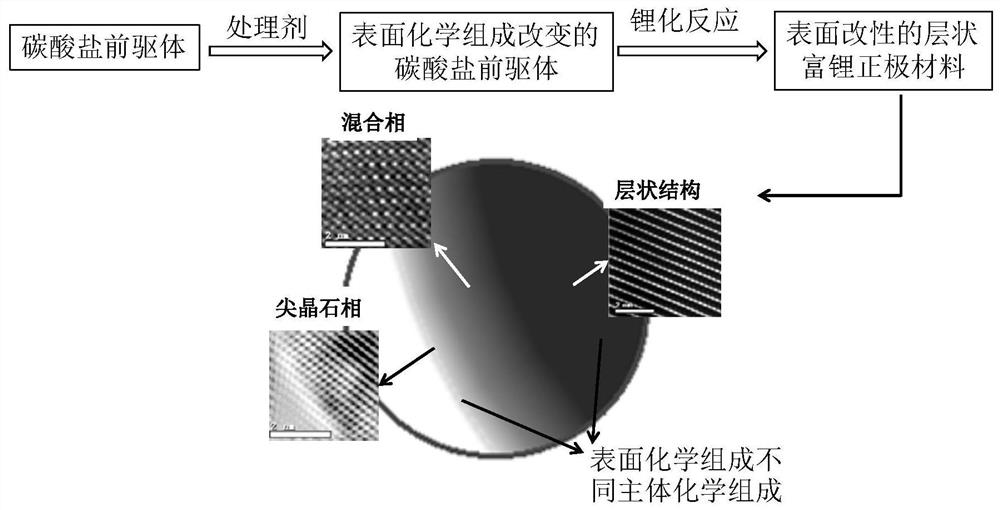
A method for simultaneous regulation of surface structure and chemical composition of layered lithium-rich cathode materials
A lithium-rich cathode material and surface structure technology, applied in structural parts, electrochemical generators, battery electrodes, etc., can solve the problems of excellent comprehensive electrochemical performance, inability to obtain, etc., achieve excellent electrochemical performance, good application prospects, Effect of stabilizing cycle life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
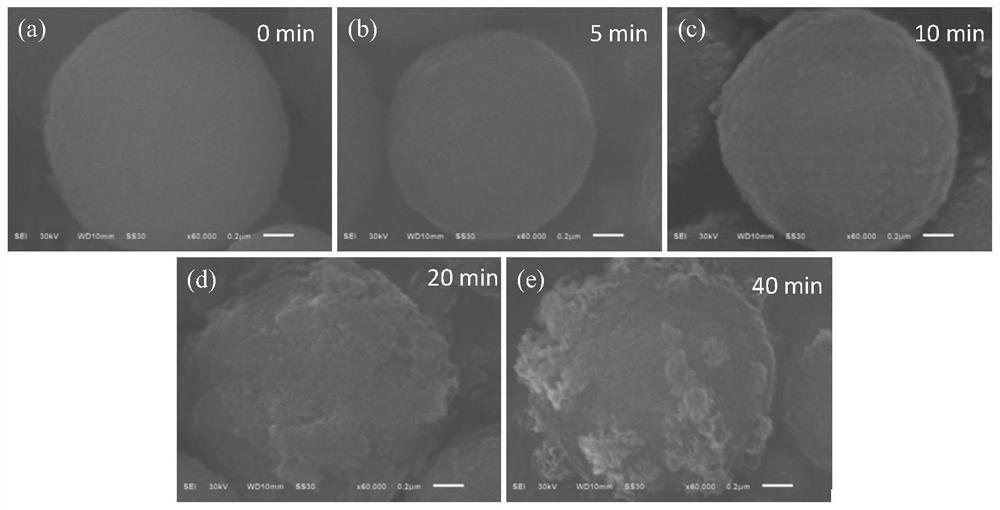
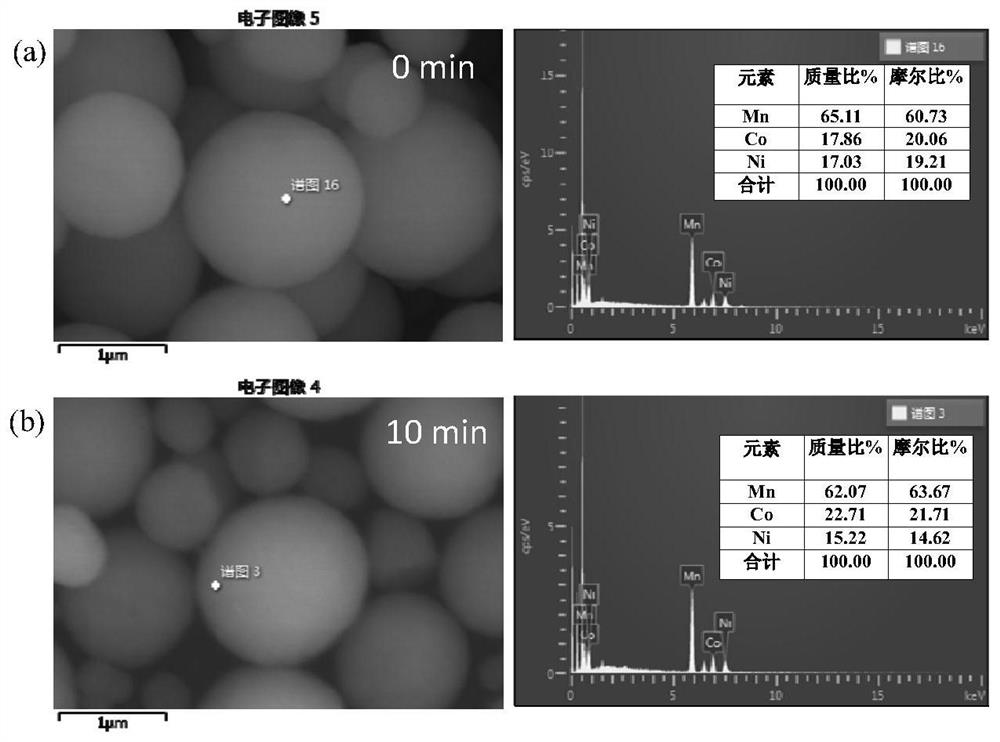
[0027] (1) Weigh the spherical Mn obtained by hydrothermal synthesis 0.6 Ni 0.2 co 0.2 CO 3 Put 2.0g of carbonate precursor in a 50mL beaker, measure 30mL of 3.0mol / L ammonia solution into the beaker containing carbonate precursor, stir magnetically for 10min, then wash and filter with distilled water several times , and finally placed in a blast drying oven at 80° C. for 12 hours to obtain a carbonate precursor treated with ammonia water. For comparison, using the same method, change the ammonia water treatment time to 5min, 20min and 40min, respectively to obtain the corresponding carbonate precursor after treatment.
[0028] (2) The carbonate precursors treated with ammonia water obtained in step (1) for 5 min, 10 min, 20 min, and 40 min were respectively calcined at 500° C. for 6 h to obtain oxides.
[0029](3) Mix the oxides obtained in the above steps with lithium carbonate respectively in the ratio of [Li]:[M]=1.42:1 ([Li] is the number of moles of lithium, [M] is t...
Embodiment 2
[0036] (1) Weigh the spherical Mn obtained by hydrothermal synthesis 0.6 Ni 0.2 co 0.2 CO 3 Put 2.0g of the carbonate precursor in a 50mL beaker, measure 30mL of 2.0mol / L ammonium sulfate solution and pour it into the beaker containing the carbonate precursor, stir it magnetically for 20min, and then wash it several times with distilled water, Filter, and finally place in a blast drying oven at 80° C. for 12 hours to obtain the treated carbonate precursor.
[0037] (2) Calcinate the untreated carbonate precursor and the ammonium sulfate solution obtained in step (1) for 20 minutes at 500° C. for 6 hours to obtain the oxide.
[0038] (3) Mix the oxide and lithium carbonate obtained in the above steps uniformly at a ratio of [Li]:[M]=1.42:1, and place them in a muffle furnace for calcination at 750° C. for 12 hours to obtain a lithium-rich cathode material.
[0039] Electrochemical tests show that the lithium-rich cathode material obtained after ammonium sulfate treatment ha...
Embodiment 3
[0041] (1) Weigh the spherical Mn synthesized by co-precipitation method 0.75 Ni 0.25 CO 3 Carbonate precursor 3.0g is placed in a 50mL beaker, and 30mL of 2.0mol / L ammonium carbonate solution is poured into the beaker containing the carbonate precursor, mechanically stirred for 30min, and then washed several times with distilled water, Filter, and finally place in a blast drying oven at 80° C. for 12 hours to obtain an ammonia-treated carbonate precursor.
[0042] (2) The untreated carbonate precursor treated with the ammonium carbonate solution obtained in step (1) for 30 minutes was calcined at 400° C. for 8 hours to obtain an oxide precursor.
[0043] (3) Mix the oxide precursor and lithium hydroxide obtained in the above steps uniformly at a ratio of [Li]:[M]=1.55:1, and place them in a muffle furnace for calcination at 700°C for 24 hours to obtain a lithium-rich positive electrode Material.
[0044] Electrochemical tests show that the lithium-rich cathode material ob...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
| retention rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com