Immunomagnetic composition, its preparation method, use and kit for treating cancer
A composition and magnetic technology, applied in the direction of immunoglobulin, organic active ingredients, chemical instruments and methods, etc., can solve the problems of consumables, inability to circulate into the target area, limit dose, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0084] 1.2 Preparation of Examples 1-3
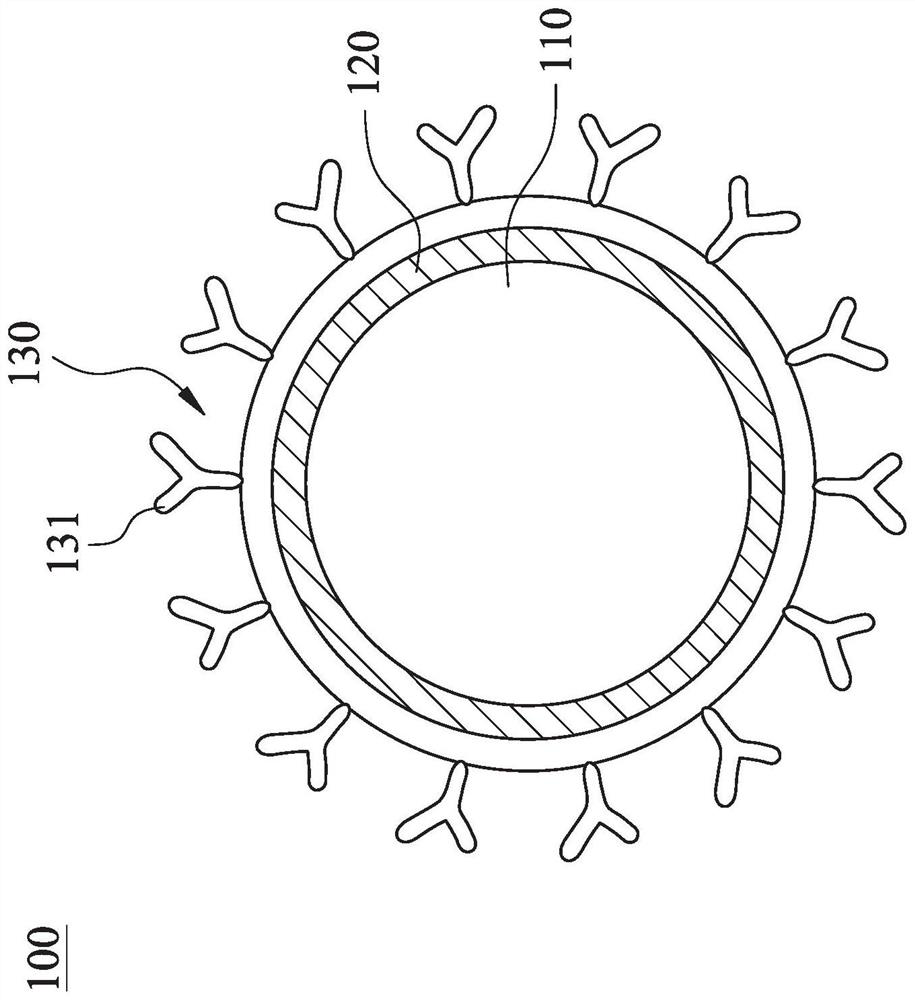
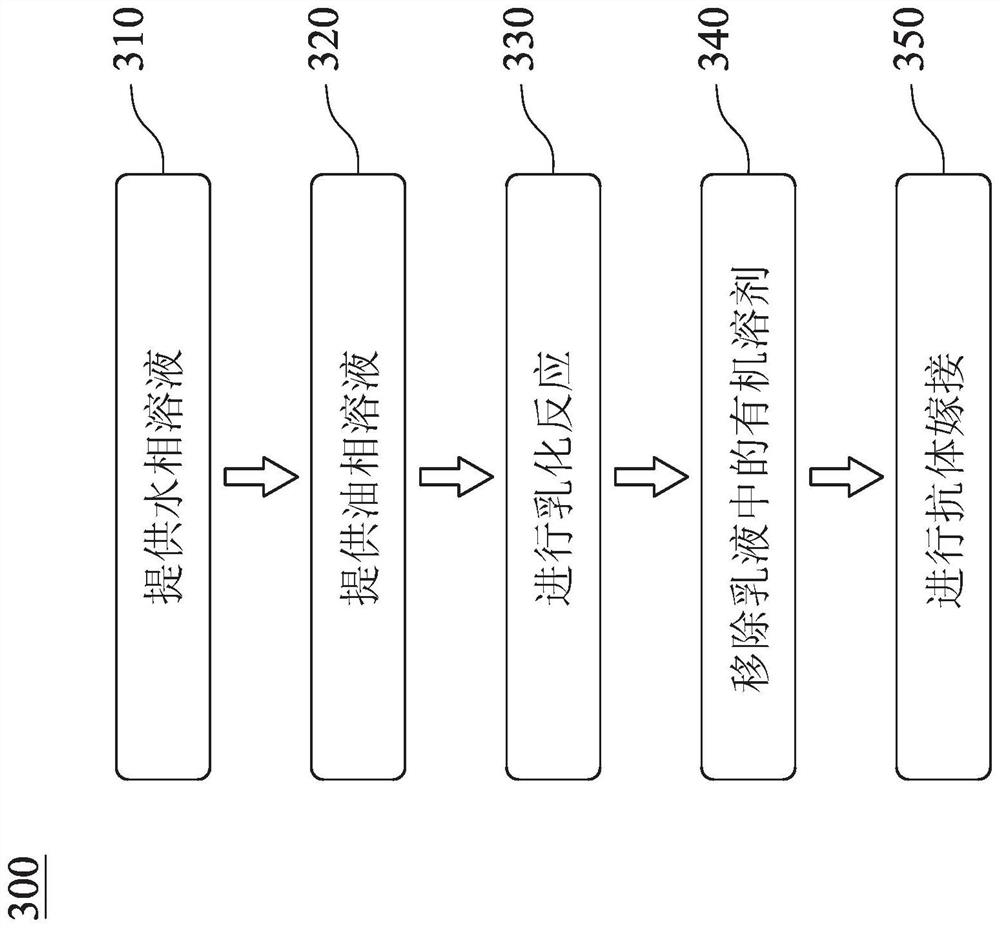
[0085] The immunomagnetic composition of the present invention is further prepared under the aforementioned conditions for optimal preparation of IO@FuDex. The prepared IO@FuDex was grafted with the buffer solution (0.1M, pH=6) containing different antibodies and containing sodium cyanoborohydride (5μM) at 4°C for 4-6 hours, the aldehyde group in Dex The Schiff base will be formed with the primary amine on the antibody, and after the reductive amination reaction using sodium cyanoborohydride, the grafted antibody will reach a chemically stable state. Finally, the prepared immunomagnetic composition is purified using magnetic separation equipment. Please refer to Table 3 below, which are the antibodies used in Examples 1-3 of the present invention, wherein the antibodies used in Example 1 are CD3 antibodies and CD8 antibodies, and the antibodies used in Example 2 are PD-L1 antibodies. 3 The antibodies used are PD-L1 antibody, CD3 antib...
Embodiment 2 test 。 pic 11B Embodiment 2 and IgGIO@FuDex test 。 pic 11CIO@FuDex no. 1、4、12 and 24 and 4T1 pic 。 pic 11D Embodiment 2 no. 1、4、12 and 24 and 4T1 pic 。 pic 11E1.4μg/ml(;L)、7μg/ml(;M) and 35μg/ml(;H)CD3/CD28 Embodiment 3
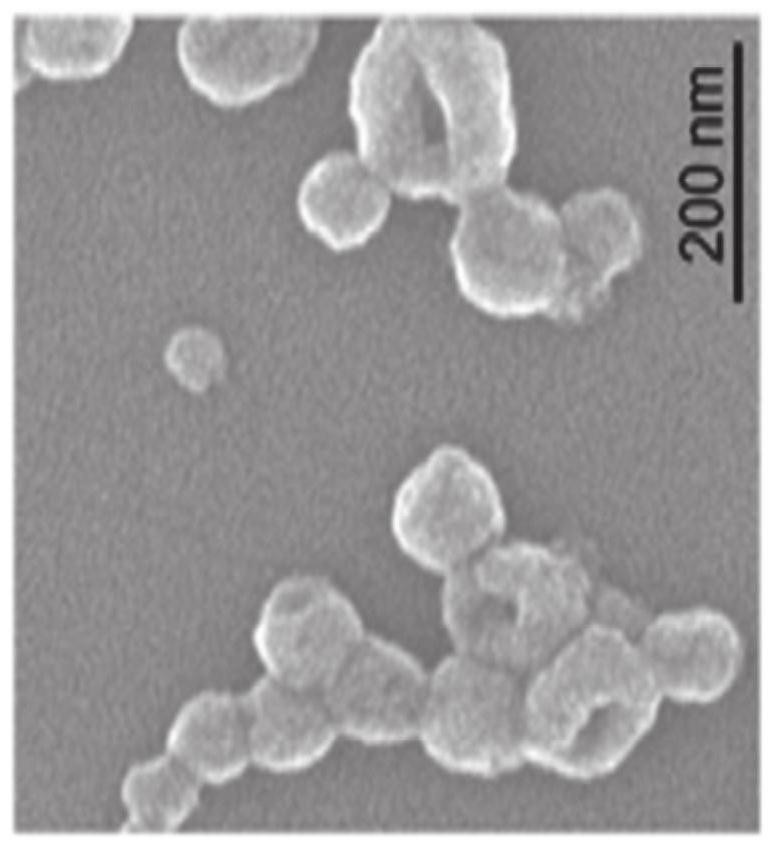
[0098] Please refer to Figure 11A to Figure 11F , is an analysis result diagram of the targeting ability and cell binding ability of the immunomagnetic composition of the present invention. in Figure 11A In vitro targeting ability test of Example 2 of PD-L1 antibodies with grafting concentrations of 1.4 μg / ml (low concentration; L), 7 μg / ml (medium concentration; M) and 35 μg / ml (high concentration; H) result. Figure 11B It is the test result of the in vitro targeting ability of IO@FuDex grafted with IgG in Example 2. Figure 11C It is the analysis results of IO@FuDex binding ability to 4T1 cell line at 1, 4, 12 and 24 hours. Figure 11D It is the analysis result diagram of the binding ability of Example 2 to 4T1 cell line at 1, 4, 12 and 24 hours. Figure 11E Example 3 of CD3 antibody / CD28 antibody with a grafting concentration of 1.4 μg / ml (low concentration; L), 7 μg / ml (medium concentration; M) and 35 μg / ml (high concentration; H) in vitro targeting ability Test Re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap