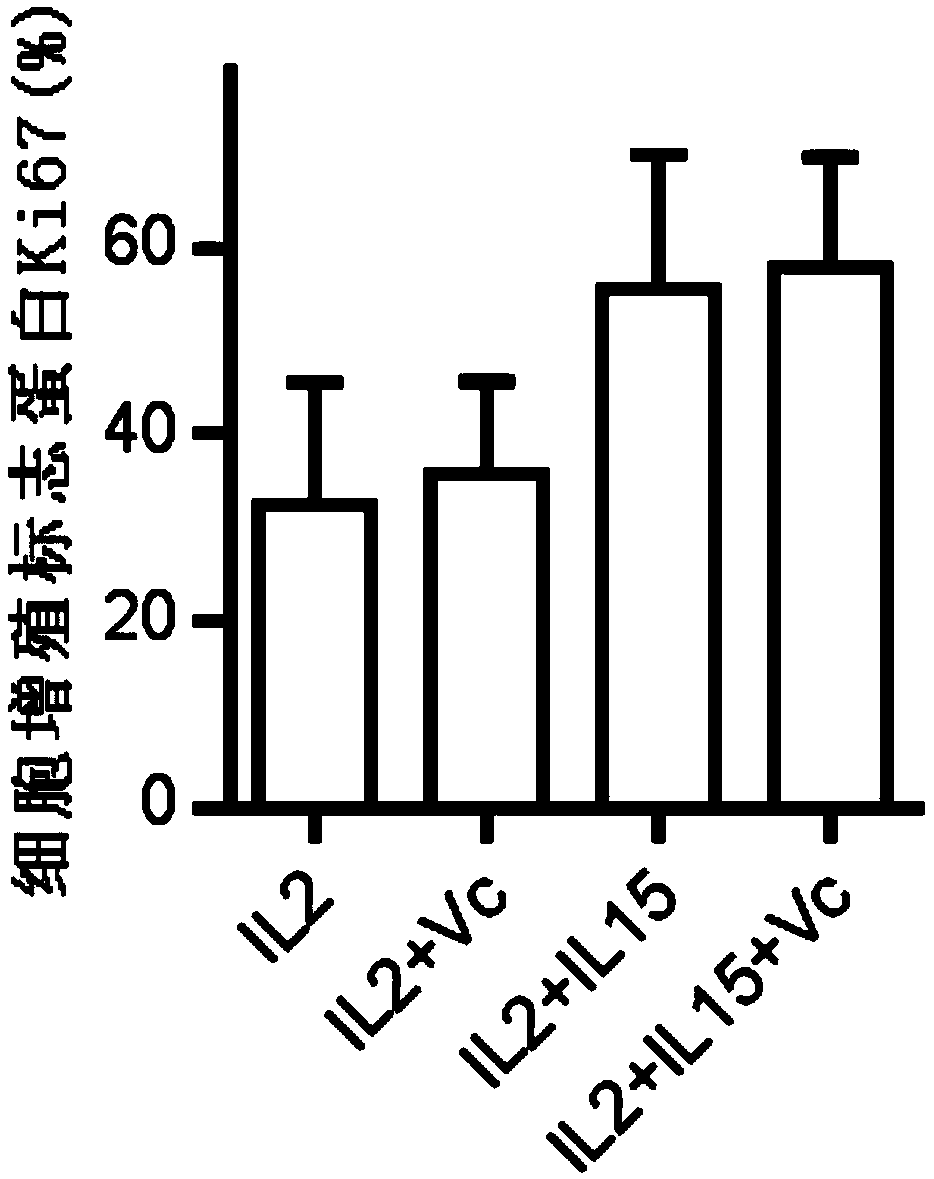
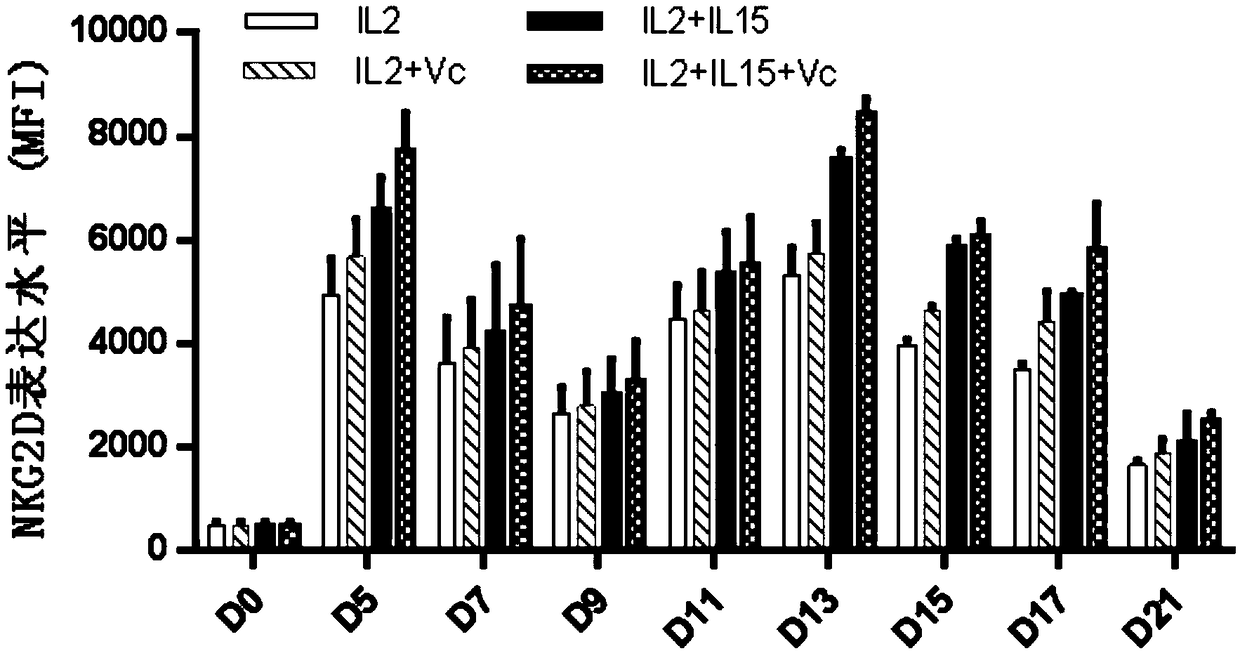
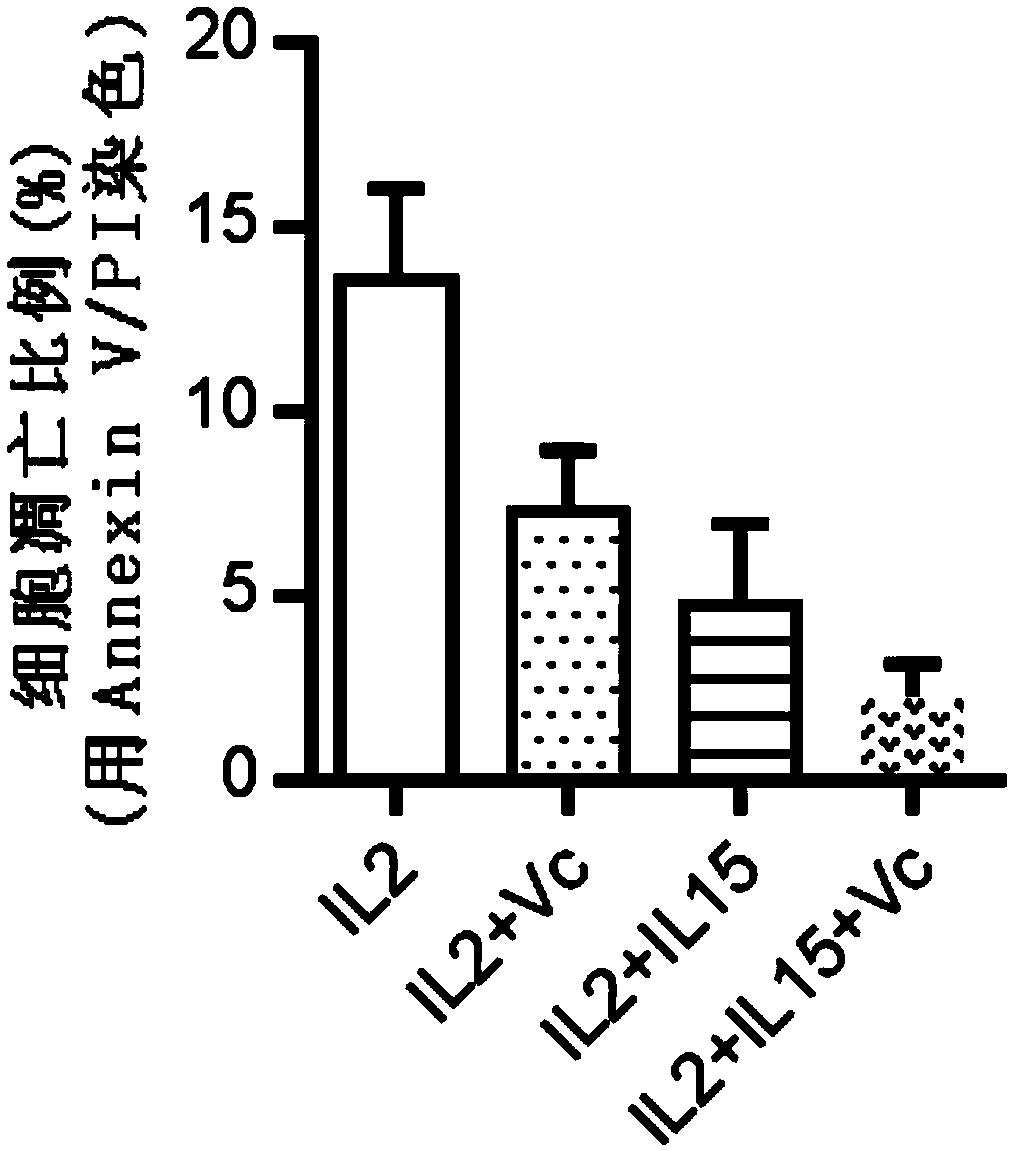Human Vgamma9Vdelta2T cell proliferation method and culture medium
A cell culture and culture medium technology, applied in the field of human Vγ9Vδ2T cell expansion method and culture medium, can solve the problems of short survival time, weak tumor cell killing ability, weak anti-apoptosis ability of cells, etc., and achieve long survival time , strong killing and inhibiting ability, strong anti-apoptotic ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] This embodiment 1 provides a human Vγ9Vδ2 T cell culture medium, including RPMI-1640 medium and interleukin-2, interleukin-15 and vitamin C added to the RPMI-1640 medium.
[0104] Wherein, in the human Vγ9Vδ2T cell culture medium, the concentration of interleukin-2 is 300 ng / ml, the concentration of interleukin-15 is 500 ng / ml, and the concentration of vitamin C is 100 mM.
Embodiment 2
[0106] This embodiment 2 provides a human Vγ9Vδ2 T cell culture medium, including RPMI-1640 medium and interleukin-2, interleukin-15 and vitamin C added to the RPMI-1640 medium.
[0107] Wherein, in the human Vγ9Vδ2T cell culture medium, the concentration of interleukin-2 is 500 ng / ml, the concentration of interleukin-15 is 700 ng / ml, and the concentration of vitamin C is 300 mM.
Embodiment 3
[0109] This embodiment 3 provides a human Vγ9Vδ2T cell stimulation and expansion medium, including the human Vγ9Vδ2T cell culture medium as described in Example 1 and zoledronic acid added to the human Vγ9Vδ2T cell culture medium.
[0110] Wherein, the concentration of zoledronic acid in the human Vγ9Vδ2T cell stimulation expansion medium is 300 μM.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com