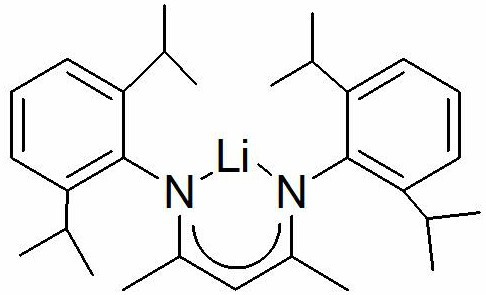
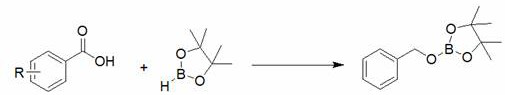
Method for preparing boric acid ester based on anilinolithium compound
A technology for lithium anilide and compound, which is applied in the field of preparing boric acid ester based on lithium anilide compound, can solve the problems of high cost, difficult catalyst, high safety risk and the like, and achieves short reaction time, high reaction yield and high catalytic activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment one: Lithium anilide catalyzes the hydroboration reaction of benzoic acid and pinacol borane
[0024] Under an inert gas atmosphere, add benzoic acid (61.1 mg, 0.5 mmol) to the dehydrated and deoxygenated reaction flask, add pinacol borane (218 μL, 1.5 mmol) with a pipette gun, and finally add 40 μl Tetrahydrofuran solution (0.1M) of lithium anilide (0.8 mol% dosage, the same below), reacted at room temperature for 75 minutes, the reaction solution was exposed to air to terminate the reaction, and the solvent was removed under reduced pressure to obtain the product boric acid ester, which was decomposed by methoxy Phenylbenzene (84.15 mg, 0.5 mmol) was used as internal standard, and CDCl 3 Dissolved, stirred for 10 minutes, sampled, and NMR. Calculated 1 H yield is 99%; 3 (THF) 2 , no product is obtained. NMR data of the product: 1 H NMR (400 MHz, CDCl 3 ) : δ7.20– 7.30 (m, 5H, ArH), 4.91 (s, 2H, CH 2 ), 1.24 (s, 36H, CH 3 ).
[0025] When the amoun...
Embodiment 2
[0031] Embodiment 2: Lithium anilide catalyzes the hydroboration reaction of 4-fluorobenzoic acid and pinacol borane
[0032] Under an inert gas atmosphere, add 4-fluorobenzoic acid (70.8 mg, 0.5 mmol) to the reaction flask after dehydration and deoxygenation treatment, add pinacol borane (290 μL, 2 mmol) with a pipette gun, and finally add A THF solution of lithium anilide (0.8 mol% consumption) was reacted at room temperature for 75 minutes. mmol) as internal standard, with CDCl 3 Dissolved, stirred for 10 minutes, sampled, and NMR. Calculated 1 H yield was 91%. NMR data of the product: 1 H NMR (400 MHz, CDCl 3 ): δ7.20– 7.30 (m, 5H, ArH), 4.91 (s, 2H, CH 2 ), 1.24 (s, 36H, CH 3 ).
Embodiment 3
[0033] Embodiment three: Lithium o-methylanilide catalyzes the hydroboration reaction of 4-bromobenzoic acid and pinacol borane
[0034] Under an inert gas atmosphere, add 4-bromobenzoic acid (100 mg, 0.5 mmol) to the reaction flask after dehydration and deoxygenation treatment, add pinacol borane (289 μL, 2 mmol) with a pipette gun, and finally add o- The tetrahydrofuran solution of methylanilinide lithium (0.8mol% consumption) was reacted at room temperature for 75 minutes, and the reaction solution was exposed to air to terminate the reaction, and the solvent was removed under reduced pressure to obtain the product boric acid ester. , 0.5 mmol) as internal standard, with CDCl 3 Dissolved, stirred for 10 minutes, sampled, and NMR. Calculated 1 H yield was 93%. NMR data of the product: 1 H NMR (400 MHz, CDCl 3 ): δ7.39 (br s, 2H, ArCH), 7.16 (t, 2H, ArCH), 4.80 (s, 2H, OCH 2 ), 1.19 (s, 36H,CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com