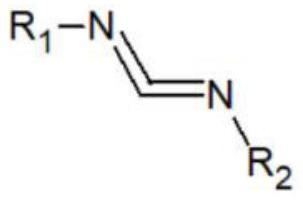
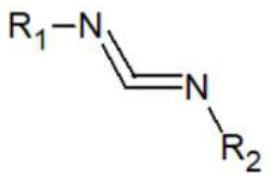
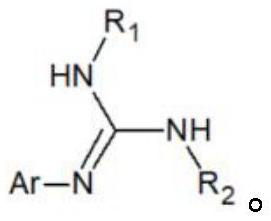
Catalyst for amination synthesis of carbodiimide, synthesis method and obtained guanidyl compound
A technology for carbodiimide amine and amine-based compounds, which is applied in the field of guanidine-based compound synthesis, can solve problems such as inability to use on a large scale, poor reaction economy, high cost and the like, and achieves the advantages of being beneficial to large-scale industrial production, short reaction time, The effect of low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Reaction of Aniline with N,N-Diisopropylcarbodiimide Catalyzed by Lithium Triethylborohydride
[0049] In the glove box, take 0.09313 g (1 mmol) of aniline and 0.1262 g (1 mmol) of N,N-diisopropylcarbodiimide into a dry reaction flask, and then add 0.005 ml of 1mol / L tris The tetrahydrofuran solution of lithium ethyl borohydride was stirred at room temperature for 0.5 hours, left open in the air for 0.5 hours to stop the reaction, added 3 ml of n-hexane to dissolve, and recrystallized to obtain the product with a yield of 99%. And carbon spectrum confirms that gained compound is target compound, as follows:
[0050] 1 HNMR (400MHz, CDCl3) δ = 7.17 (d, J = 7.5Hz, 1H), 6.86 (t, J = 7.4Hz, 1H), 6.82–6.76 (m, 2H), 3.70 (s, 2H), 3.49 ( s,1H),1.10(d,J=6.4Hz,12H);
[0051] 13 C NMR (101MHz, CDCl3) δ=150.32, 150.11, 129.25, 123.54, 121.33, 43.21, 23.36.
Embodiment 2
[0053] Reaction of p-Bromoaniline with N,N-Diisopropylcarbodiimide Catalyzed by Lithium Triethylborohydride
[0054] Take 0.1720 g (1 mmol) of p-bromoaniline and 0.1262 g (1 mmol) of N,N-diisopropylcarbodiimide in the glove box and add it to a dry reaction flask, then add 0.005 ml of 1mol / L with a syringe The tetrahydrofuran solution of lithium triethylborohydride was stirred at room temperature for 0.5 hours, and left open in the air for 0.5 hours to stop the reaction, added 5 milliliters of n-hexane to dissolve, and recrystallized to obtain the product with a yield of 99%. Proton spectrum and carbon spectrum confirm that gained compound is target compound, as follows:
[0055] 1 HNMR (400MHz, CDCl3) δ=7.11(d, J=8.6Hz, 2H), 6.70(d, J=8.6Hz, 2H), 3.67(dt, J=12.7, 6.3Hz, 2H), 3.50–3.45( m,2H),1.08(d,J=6.4Hz,12H);
[0056] 13 C NMR (101 MHz, CDCl3) δ = 149.08, 148.60, 131.11, 124.31, 112.74, 42.13, 22.28.
Embodiment 3
[0058] Reaction of o-Chloroaniline with N,N-Diisopropylcarbodiimide Catalyzed by Lithium Triethylborohydride
[0059] In the glove box, take 0.1720 g (1 mmol) of o-chloroaniline and 0.1262 g (1 mmol) of N,N-diisopropylcarbodiimide into a dry reaction flask, and then add 0.005 ml of 1mol / L with a syringe The tetrahydrofuran solution of lithium triethylborohydride was stirred at room temperature for 0.5 hours, and left open in the air for 0.5 hours to stop the reaction, added 5 milliliters of n-hexane to dissolve, and recrystallized to obtain the product with a yield of 99%. Proton spectrum and carbon spectrum confirm that gained compound is target compound, as follows:
[0060] 1 HNMR (400MHz, CDCl3) δ7.28(dd, J=7.9, 1.3Hz, 1H), 7.07(td, J=7.8, 1.4Hz, 1H), 6.87–6.77(m, 2H), 3.79–3.63(m ,2H),1.12(d,J=6.4Hz,12H);
[0061] 13 C NMR (101 MHz, CDCl3) δ = 156.87, 154.46, 150.71, 137.77, 126.21, 124.52, 122.20, 116.06, 43.31, 23.31.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com