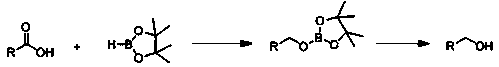N-butyl lithium based fatty alcohol preparation method
A technology of aliphatic alcohol and n-butyllithium, which is applied in the direction of hydrolysis preparation, chemical instruments and methods, and compounds containing elements of group 3/13 of the periodic table, etc., can solve the problem of high cost and achieve short reaction time and simple reaction Controllable, simple and controllable effect of the reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: N-butyllithium catalyzes the hydroboration reaction of acetic acid and pinacol borane
[0025] Under an inert gas atmosphere, add acetic acid (28.6 μL, 0.5 mmol) to the reaction flask after dehydration and deoxygenation treatment, add pinacol borane (290 μL, 2 mmol) with a pipette gun, and finally add 10 μL n-butyl Lithium tetrahydrofuran solution (0.1M) (0.2 mol% dosage, the same below), hydroboration reaction at room temperature for 15 minutes, the reaction solution was exposed to the air to obtain the product borate, with s-trimethoxybenzene (84.08 mg, 0.5 mmol) as internal standard, with CDCl 3 Dissolved, stirred for 10 minutes, sampled, and NMR. Calculated 1 The yield of H was 99%, when pinacol borane (218 μL, 1.5 mmol), the yield was 95%; when pinacol borane (363 μL, 2.5 mmol), the yield was 99%; the NMR data of the product : 1 H NMR (400 MHz, CDCl 3 ): δ3.85(q, 2H, CH 2 ), 1.23 (s, 36H, CH 3 ), 1.21 (br s, 3H, CH 3 ). If n-butyllithium is r...
Embodiment 2
[0029] Example 2: n-Butyl Lithium Catalyzed Hydroboration Reaction of Valeric Acid and Pinacol Borane
[0030] Under an inert gas atmosphere, add valeric acid (54.38 μL, 0.5 mmol) to the reaction flask after dehydration and deoxygenation treatment, add pinacol borane (290 μL, 2 mmol) with a pipette gun, and finally add n-butyl The tetrahydrofuran solution of lithium (0.2 mol% dosage) was reacted at room temperature for 15 minutes, the reaction solution was exposed to air, and the solvent was removed to obtain the product borate, with s-trimethoxybenzene (84.12 mg, 0.5 mmol) as the internal standard, with CDCl 3 Dissolved, stirred for 10 minutes, sampled, and NMR. Calculated 1 H yield was 93%. NMR data of the product: 1 H NMR (400 MHz, CDCl 3 ): δ 3.81(t, 2H, OCH 2 ), 1.51-1.55 (m, 2H,CH 2 ), 1.29-1.51 (m, 4H, CH 2 ), 1.27(s, 36H, CH), 0.85(t, 3H, CH 3 ). Add 1.1g of silica gel and 3mL of methanol to the above hydroboration reaction after removing the solvent, and rea...
Embodiment 3
[0031] Embodiment 3: n-Butyllithium catalyzes the hydroboration reaction of hexanoic acid and pinacol borane
[0032]Under an inert gas atmosphere, add hexanoic acid (62.52 μL, 0.5 mmol) to the reaction flask after dehydration and deoxygenation treatment, add pinacol borane (290 μL, 2 mmol) with a pipette gun, and finally add 4-formazol The tetrahydrofuran solution of oxyn-butyllithium (0.2mol% dosage) was reacted at room temperature for 15 minutes, and the reaction solution was exposed to air, and the solvent was removed to obtain the product borate, which was prepared in the form of s-trimethoxybenzene (84.01 mg, 0.5 mmol ) as internal standard, with CDCl 3 Dissolved, stirred for 10 minutes, sampled, and NMR. Calculated 1 H yield was 90%. NMR data of the product: 1 H NMR (400 MHz, CDCl 3 ): δ 3.72 (t,2H,OCH 2 ), 1.45-1.51 (m, 2H,CH 2 ), 1.23-1.34 (m, 6H, CH 2 ), 1.17 (s, 48H, CH 3 ), 0.81 (t, 3H, CH 3 ). Add 1.1g of silica gel and 3mL of methanol to the above hydr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com