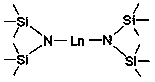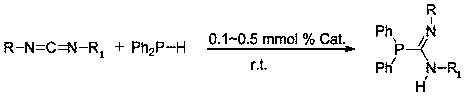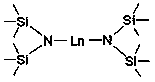Method for preparing phosphine guanidine compound
A technology for compounds and rare earth complexes, applied in the application field of metal organic complexes, can solve the problems of difficult synthesis of catalyst structures, difficult to realize industrial production, and unsatisfactory yields, and achieve simple and controllable reaction processes. Short time, high productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment one: Yb[N(SiMe 3 ) 2 ] 2 Catalytic Synthesis of Phosphineguanidine from Diphenylphosphine and N,N'-Diisopropylcarbodiimide
[0025] Under an inert gas atmosphere, add diphenylphosphine (93.1mg, 0.5mmol) to the reaction flask after dehydration and deoxygenation treatment, and then add Yb[N(SiMe 3 ) 2 ] 2 Toluene solution (0.025 mL, 0.0025 mmol), then add N,N'-diisopropylcarbodiimide (63.1mg, 0.5mmol) with a pipette gun, react at room temperature for 1h, add CDCl 3 Dubbed into a solution. Calculated 31 The P spectrum yield was 95%. The solution was dried under reduced pressure, extracted and filtered with n-hexane to obtain a colorless transparent solution, and then the solvent was dried under reduced pressure, and the reactant was recrystallized in hexane to obtain the corresponding phosphine guanidine. i PrN=C(PPh 2 )(NH i Pr).
Embodiment 2
[0026] Embodiment two: Eu[N(SiMe 3 ) 2 ] 2 Catalytic Synthesis of Phosphineguanidine from Diphenylphosphine and N,N'-Diisopropylcarbodiimide
[0027] Under an inert gas atmosphere, add diphenylphosphine (93.1mg, 0.5mmol) to the reaction flask after dehydration and deoxygenation treatment, and then add Eu[N(SiMe 3 ) 2 ] 2 Toluene solution (0.025 mL, 0.0025 mmol), then add N,N'-diisopropylcarbodiimide (63.1mg, 0.5mmol) with a pipette gun, react at room temperature for 1h, add CDCl 3 Dubbed into a solution. Calculated 31 The P spectrum yield was 90%. The solution was dried under reduced pressure, extracted and filtered with n-hexane to obtain a colorless transparent solution, and then the solvent was dried under reduced pressure, and the reactant was recrystallized in hexane to obtain the corresponding phosphine guanidine. i PrN=C(PPh 2 )(NH i Pr).
Embodiment 3
[0028] Embodiment three: Sm[N(SiMe 3 ) 2 ] 2 Catalytic Synthesis of Phosphineguanidine from Diphenylphosphine and N,N'-Diisopropylcarbodiimide
[0029] Under an inert gas atmosphere, add diphenylphosphine (93.1 mg, 0.5 mmol) to the reaction flask after dehydration and deoxygenation treatment, and then add Sm[N(SiMe 3 ) 2 ] 2 Toluene solution (0.025 mL, 0.0025 mmol), then add N,N'-diisopropylcarbodiimide (63.1mg, 0.5mmol) with a pipette gun, react at room temperature for 1h, add CDCl 3 Dubbed into a solution. Calculated 31 The P spectrum yield was 92%. The solution was dried under reduced pressure, extracted and filtered with n-hexane to obtain a colorless transparent solution, and then the solvent was dried under reduced pressure, and the reactant was recrystallized in hexane to obtain the corresponding phosphine guanidine. i PrN=C(PPh 2 )(NH i Pr).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com