An assessment management system for clinical studies initiated by researchers
A management system and researcher's technology, applied in data processing applications, medical and health care resources or facilities, instruments, etc., to achieve the effect of effective promotion, high degree of intelligence, scientific and reliable evaluation management
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
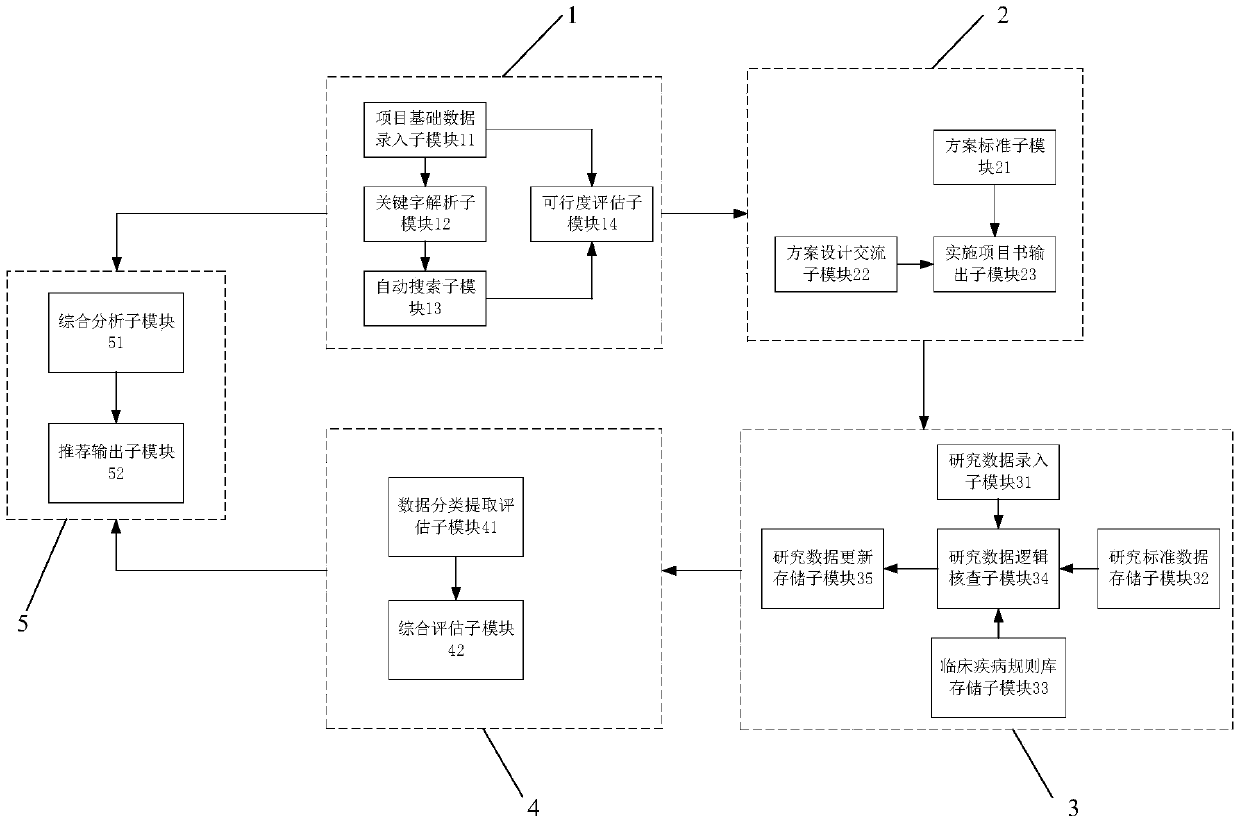
[0040] Such as figure 1 Shown, an evaluation management system for investigator-initiated clinical studies that includes:
[0041] Project evaluation module 1: This module is used to evaluate the feasibility of the clinical research initiated by the investigator;
[0042] Protocol design module 2: This module is used to design and generate the implementation protocol of clinical research;
[0043] Research data management module 3: This module is used for the collection and management of research data in the clinical research process;
[0044] Data analysis and evaluation module 4: This module is used to analyze and evaluate the collected research data and complete the evaluation of the implementation quality of the clinical research;
[0045] Project recommendation module 5: This module conducts a comprehensive analysis based on the feasibility evaluation results of project evaluation module 1 and the implementation quality evaluation results of data analysis and evaluation...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com