Method for producing hexane from aromatic hydrocarbon raffinate oil
A technology for raffinate oil and aromatics, applied in the field of isohexane and cyclohexane to produce n-hexane, which can solve the problems of low added value of products, low yield of cyclohexane, and low yield of n-hexane
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
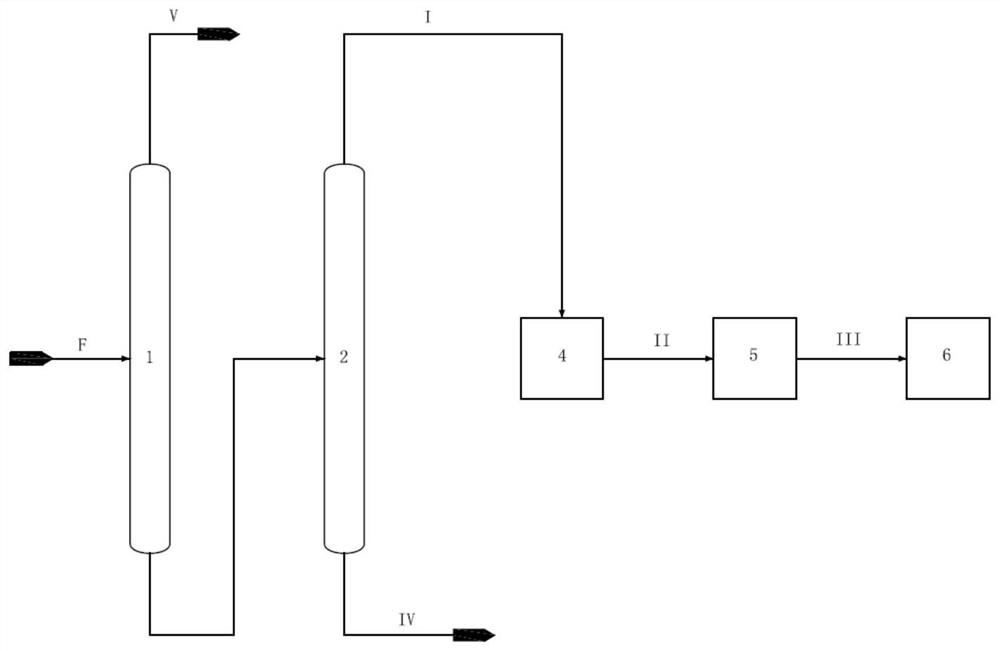
[0044] according to figure 1 Shown is a method for producing hexane from aromatics raffinate.
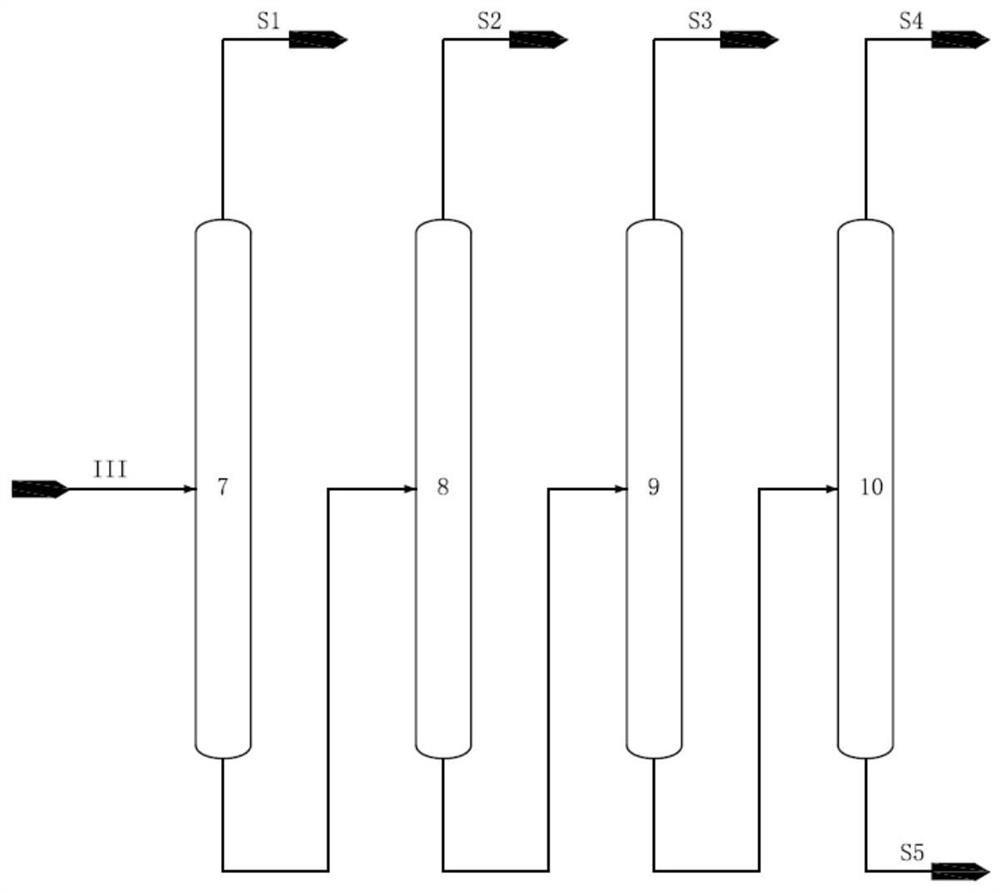
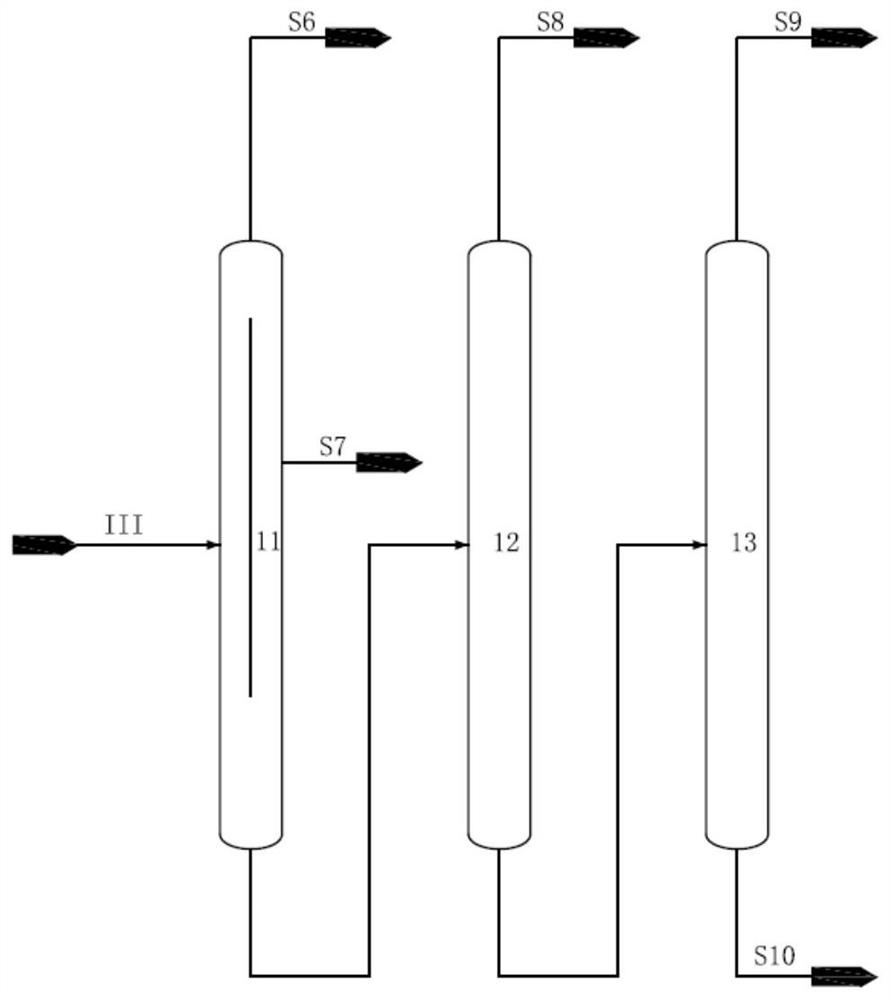
[0045] The aromatics raffinate F containing 18wt% isohexane, 10wt% normal hexane, 30wt% methylcyclopentane, 10wt% cyclohexane and 0.04wt% benzene passes through the first rectification tower and the second rectification tower successively, Obtain stream I containing isohexane, n-hexane, methylcyclopentane and benzene from the position above the top feed of the second rectification tower, and obtain C7 and above component stream IV containing cyclohexane from the position below the feed, stream I Obtain stream II through hydrogenation reactor, stream II obtains stream III containing isohexane, normal hexane, hexanaphthene through isomerization reactor, stream III passes through such as figure 2 The shown isohexane tower, n-hexane tower, methylcyclopentane tower, and cyclohexane tower are sequentially separated to obtain isohexane, n-hexane, methylcyclopentane, and cyclohexane, whe...
Embodiment 2
[0051] Implementation is similar to Example 1. The aromatics raffinate F containing 18wt% isohexane, 10wt% normal hexane, 30wt% methylcyclopentane, 10wt% cyclohexane and 0.04wt% benzene passes through the first rectification tower and the second rectification tower successively, Obtain stream I containing isohexane, n-hexane, methylcyclopentane and benzene from the position above the top feed of the second rectification tower, and obtain C7 and above component stream IV containing cyclohexane from the position below the feed, stream I Obtain stream II through hydrogenation reactor, stream II obtains stream III containing isohexane, normal hexane, hexanaphthene through isomerization reactor, stream III passes through such as figure 2 The shown isohexane tower, n-hexane tower, methylcyclopentane tower, and cyclohexane tower are sequentially separated to obtain isohexane, n-hexane, methylcyclopentane, and cyclohexane, wherein methylcyclopentane is recycled to isohexane The stru...
Embodiment 3
[0059] Implementation is similar to Example 1. The aromatics raffinate F containing 18wt% isohexane, 10wt% normal hexane, 30wt% methylcyclopentane, 10wt% cyclohexane and 0.04wt% benzene passes through the first rectification tower and the second rectification tower successively, Obtain stream I containing isohexane, n-hexane, methylcyclopentane and benzene from the position above the top feed of the second rectification tower, and obtain C7 and above component stream IV containing cyclohexane from the position below the feed, stream I Stream II is obtained through a hydrogenation reactor, and stream II is obtained through an isomerization reactor to contain stream III containing isohexane, normal hexane, and cyclohexane, and stream III passes through such as figure 2 The shown isohexane tower, n-hexane tower, methylcyclopentane tower, and cyclohexane tower are sequentially separated to obtain isohexane, n-hexane, methylcyclopentane, and cyclohexane, wherein methylcyclopentane...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap