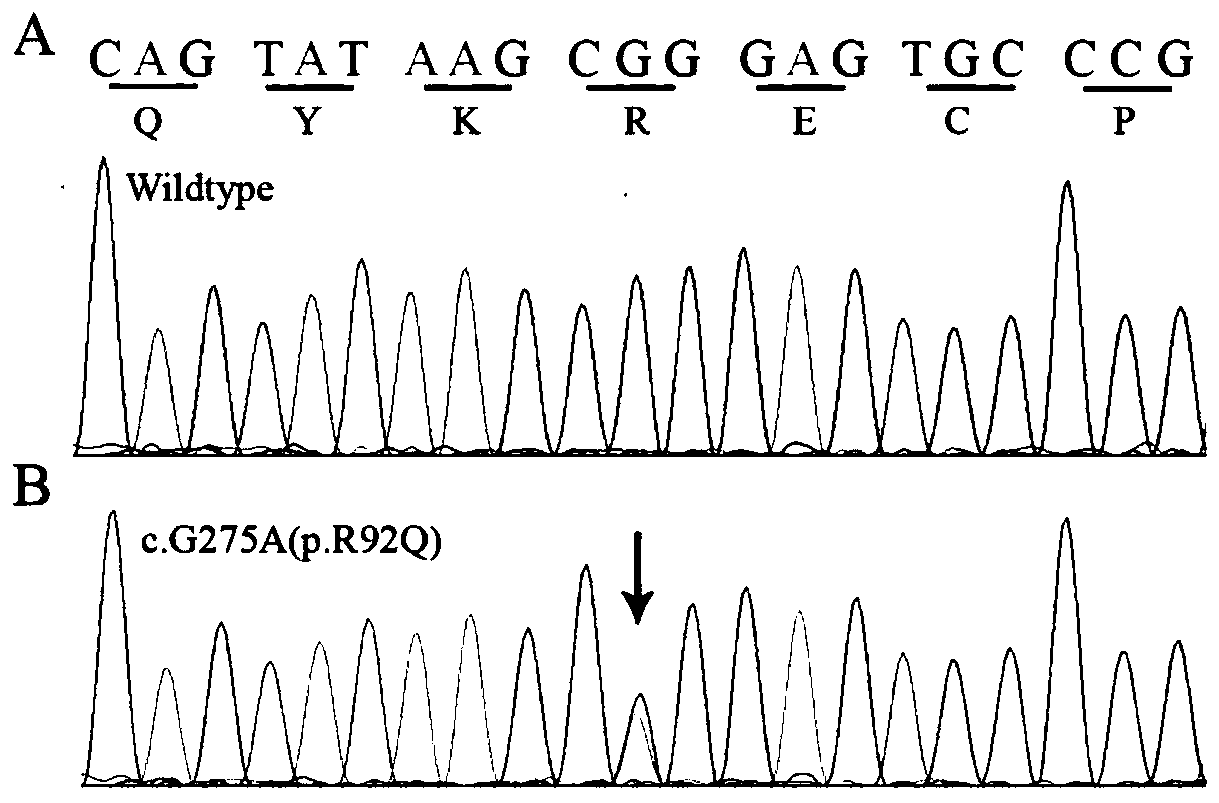
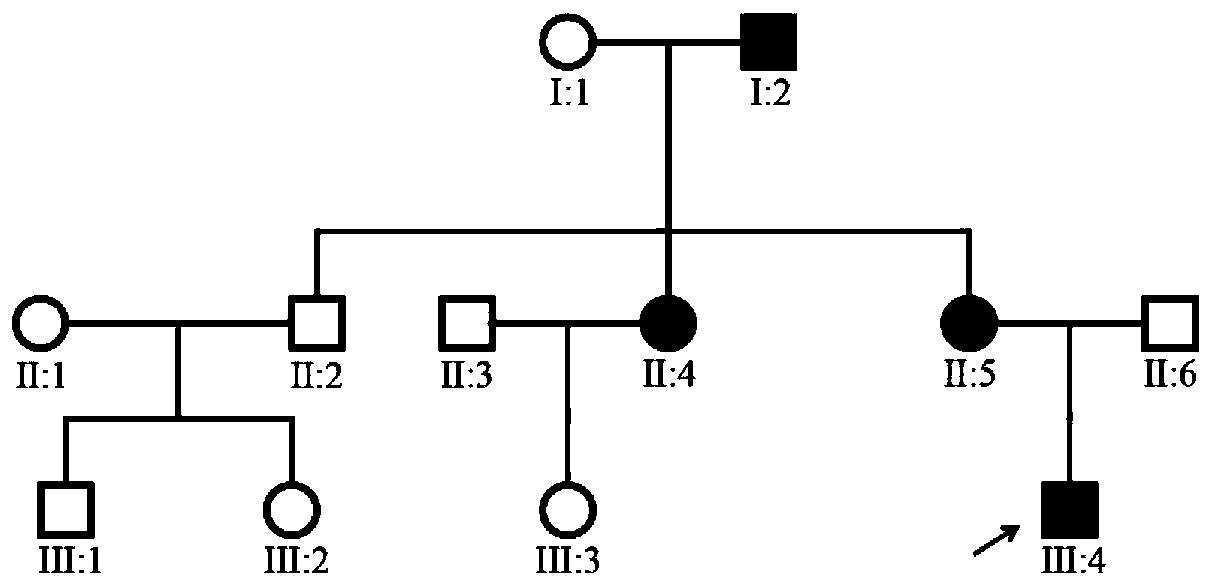
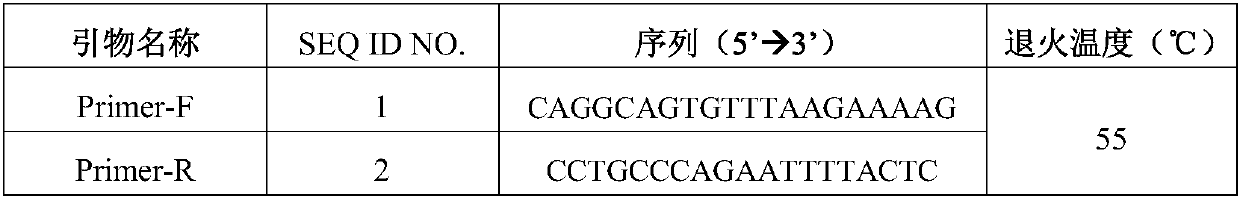
Screening kit for congenital aniridia
A kit, an irisless technology, applied in biochemical equipment and methods, microbial determination/inspection, etc., can solve problems such as false negatives, and achieve the effect of reliable, specific and accurate detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0023] Example The kit and method of use of the present invention
[0024] All components, contents and methods of use in the kit of the present invention are as follows:
[0025] 1. PCR amplification reagents (50 servings):
[0026] The PCR amplification reagent is used to amplify a piece of DNA sequence where the SNP site is located, and its composition is shown in Table 1.
[0027] Table 1 PCR amplification reagents
[0028] Component
concentration
volume
PCR mix
2×
600μl
Primer pair
10μM
100μl
Pure water
2ml
[0029] The PCR mixture in Table 1 includes Taq enzyme, dNTP, magnesium ion and other components required for conventional PCR; the primer pair information is shown in Table 2.
[0030] Table 2 Primers used for PAx6 gene amplification
[0031]
[0032] 2. PAX6 gene mutation typing detection reagent (50 people):
[0033] The reagent includes the components shown in Table 3.
[0034] Table 3 PAx6 gene mutation typing detection reagents
[0035] Component
volume
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com