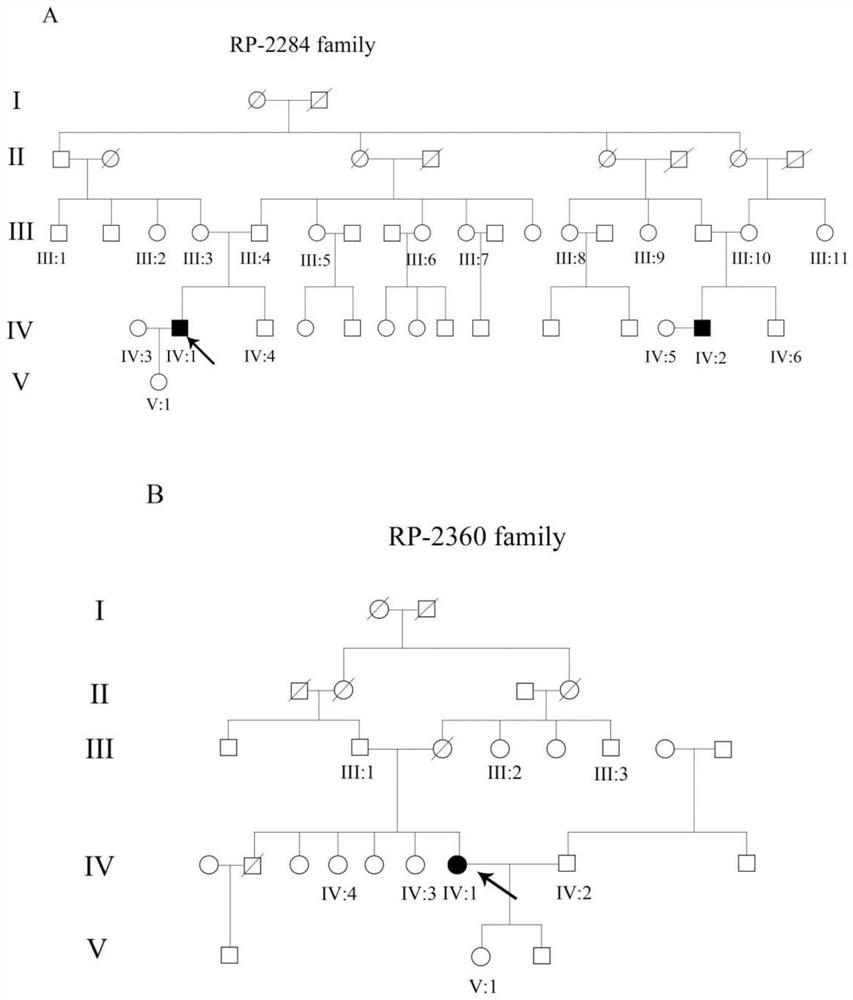
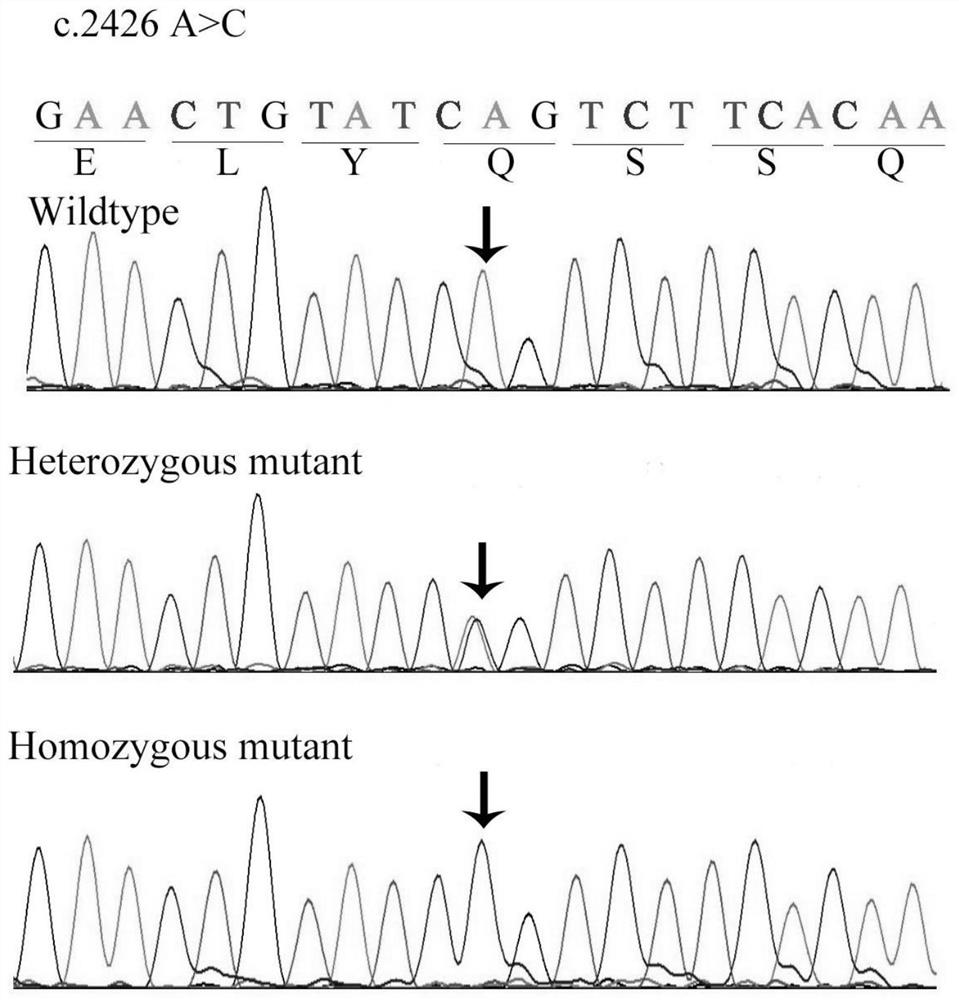
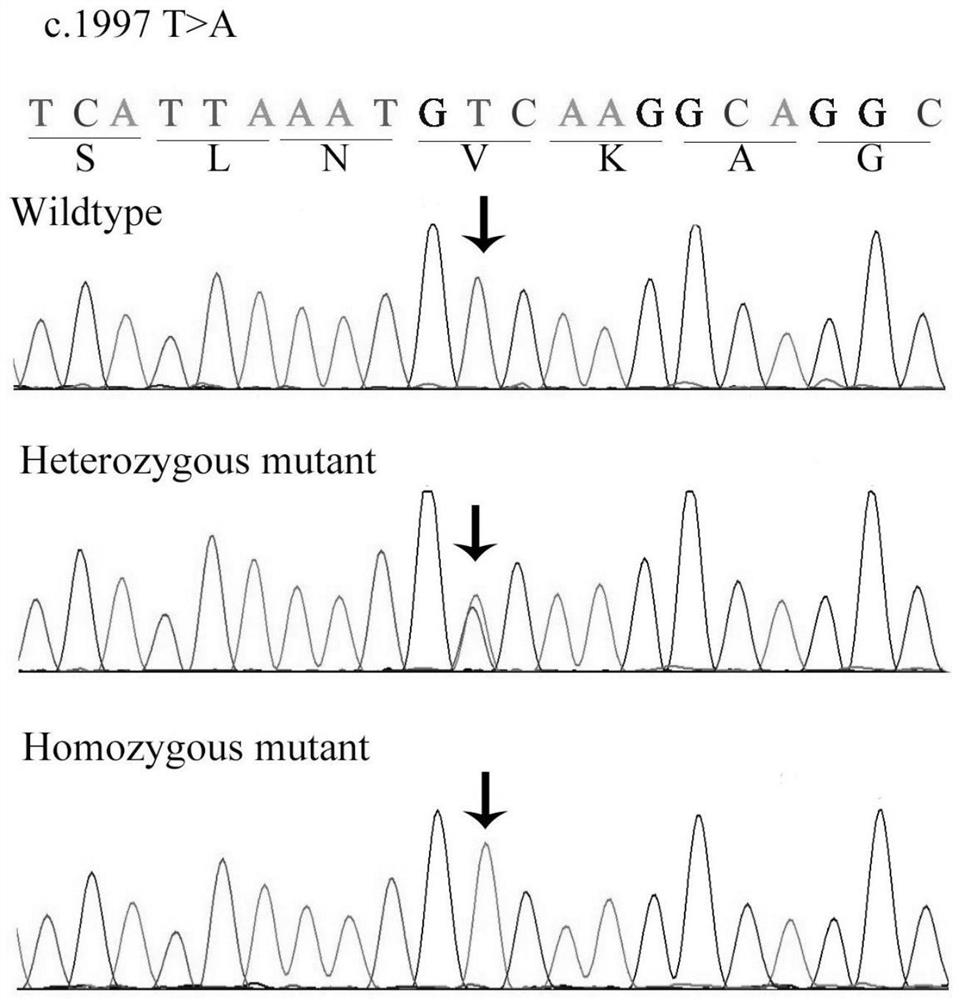
A retinitis pigmentosa screening kit
A retinal pigment and screening technology, which is applied in the field of gene point mutations and point mutations, can solve the problems of incomplete mutation sites and missed diagnosis of RP, and achieve reliable and specific effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0025] Example Kit of the present invention and method of use
[0026] All components, contents and use methods in the kit of the present invention are as follows:
[0027] 1. PCR amplification reagents (50 copies):
[0028] The PCR amplification reagent is used to amplify a DNA sequence where the mutation site is located, and its composition is shown in Table 1.
[0029] Table 1 PCR amplification reagents
[0030] component concentration volume PCR mix 2× 600μl primer pair 10μM 100μl pure water 2ml
[0031] The PCR mixture in Table 1 includes Taq enzyme, dNTP, magnesium ions and other components required for conventional PCR; the primer pair information is shown in Table 2.
[0032] Table 2 Primers used for CRB1 gene amplification
[0033]
[0034] 2. CRB1 gene variant typing detection reagent (50 copies):
[0035] The reagent included the components shown in Table 3.
[0036] Table 3 Detection reagents for CRB1 gene variant...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com