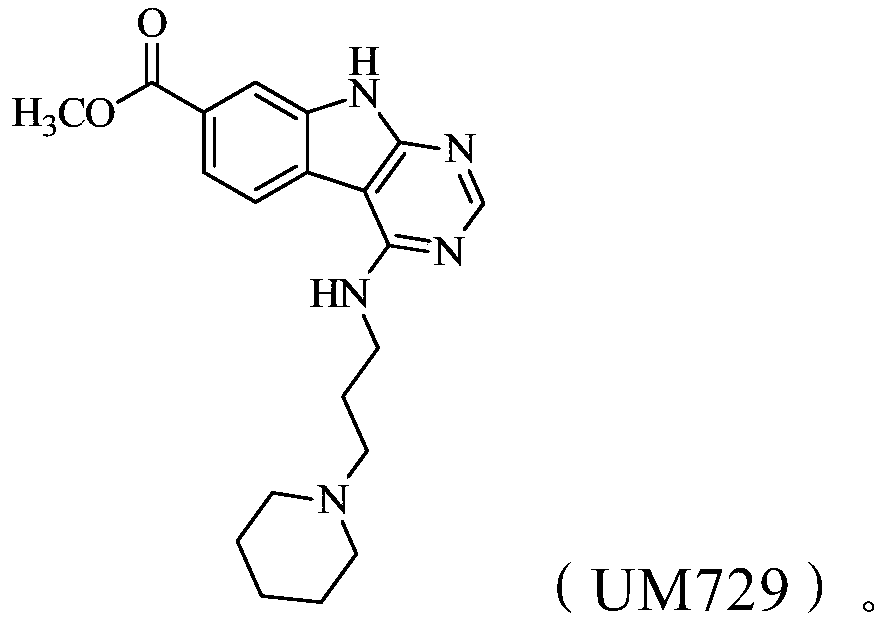
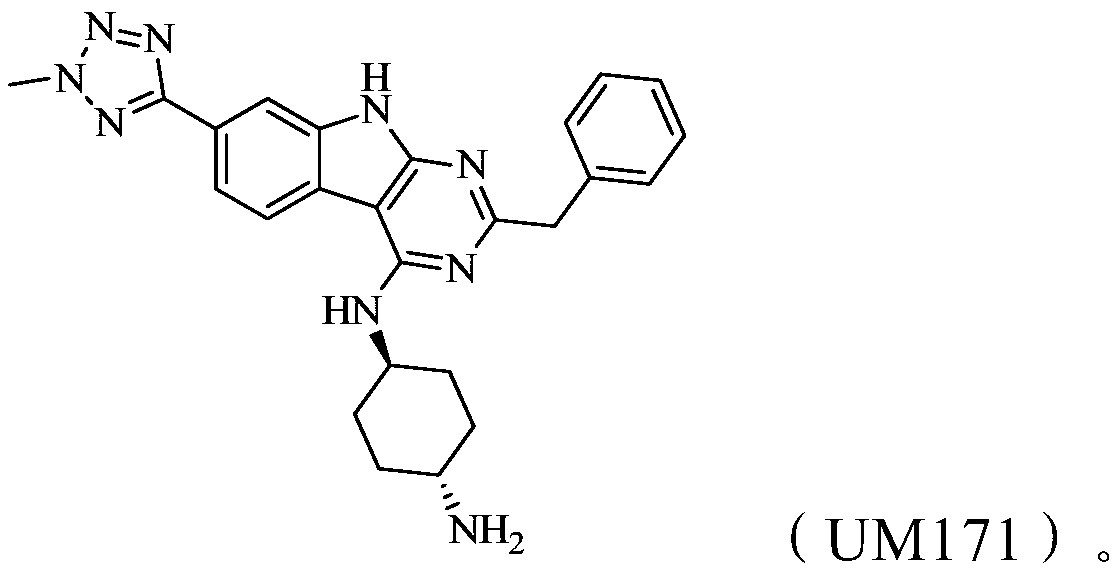
Methods of treating acute myeloid leukemia and multiple myeloma using natural killer cells
A natural killer cell and acute myeloid technology, applied in cell culture active agents, biochemical equipment and methods, animal cells, etc., can solve problems such as difficult to maintain tumor targeting and tumor killing ability, and difficult immunotherapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0610] 6.1 Example 1: Clinical Study - Acute Myeloid Leukemia
[0611] A phase I, phase I, three-phase infusion of human cord blood-derived, culture-expanded natural killer cell infusions and subcutaneous recombinant human IL-2 (rhIL-2) in adults with relapsed and / or refractory acute myeloid leukemia. Multicenter, open-label, dose-escalation safety study.
[0612] The screening / baseline period was defined as the 21 days from Day -28 to Day -7 prior to three-stage NK cell administration, during which time subjects were assessed for eligibility. The Screening / Baseline period was followed by a 5-day conditioning treatment period consisting of cyclophosphamide (60 mg / kg x 2 days on Days -5 and -4, but omitted if less than 4 months from previous transplant Cyclophosphamide dose on day -4) and fludarabine (25 mg / m 2 ×5 days, commencing on day -6) (study days -6 to -2). If less than four months have passed since the previous transplant, omit the next-day fludarabine.
[0...
Embodiment 2
[0628] Example 2: Clinical Study - Multiple Myeloma
[0629] Human cord blood-derived, culture-expanded three-stage natural killer cell infusion and subcutaneous recombinant human IL-2 (rhIL-2) following autologous stem cell transplantation (ASCT) in adults with multiple myeloma (MM) ) Phase I, multicenter, open-label safety study.
[0630] The primary objective of this study was to evaluate the safety of three-stage NK cell administration after ASCT and to determine the maximum tolerated dose in MM subjects. A secondary objective was to explore potential clinical efficacy up to day 100.
[0631] The maximum tolerated dose of three-phase natural killer cells was evaluated on days 2 and 7 after ASCT and determined on day 14 after ASCT. Three phases of natural killer cells were administered intravenously, followed by a total of six IL-2 injections to support NK cells in the body.
[0632] The primary outcome measures were adverse events and dose-limiting toxicities, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com