Methods and compositions for inducing hematopoietic cell differentiation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
hiPSC Generation and Maintenance
[0314]Somatic cells including fibroblast and blood cells were induced to reprogram towards a pluripotent state using various factor combinations including OCT4 / SOX2 / LargeT, OCT4 / SOX2 or OCT4 / SOX2 / NANOG / LargeT in the presence of reprogramming medium containing ROCK, MEK, GSK3 pathway and TGFβ receptor inhibitors (Valamehr et al. Sci Rep. 2012; 2: 213). Fourteen days after induction, reprogramming populations were switched to maintenance medium containing ROCK, GSK3, and MEK pathway inhibitors, basic fibroblast growth factor (bFGF), and leukemia inhibitory factor (LIF) (Valamehr et al. Stem Cell Reports 2014, 2(3): 366-381). Cells were kept indefinitely in the maintenance medium.
[0315]Approximately three weeks after induction, the reprogramming populations were sorted into individual wells of a 96-well plate. Selected clones were characterized and fully reprogrammed clones representative of naïve hiPSCs were selected for differentiation studies (Valameh...
example 2
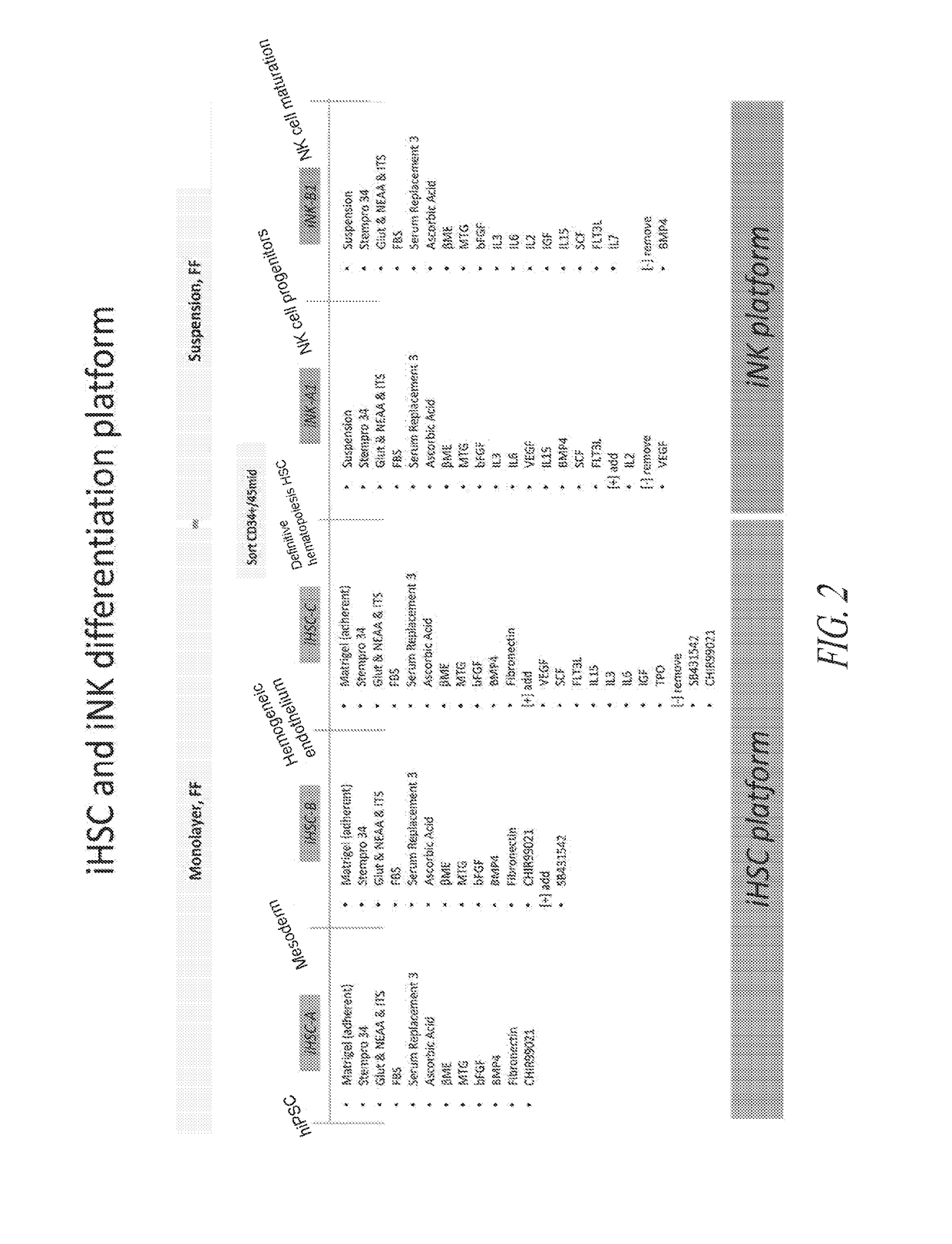
Hematopoietic Differentiation Using iHSC Culture Platform
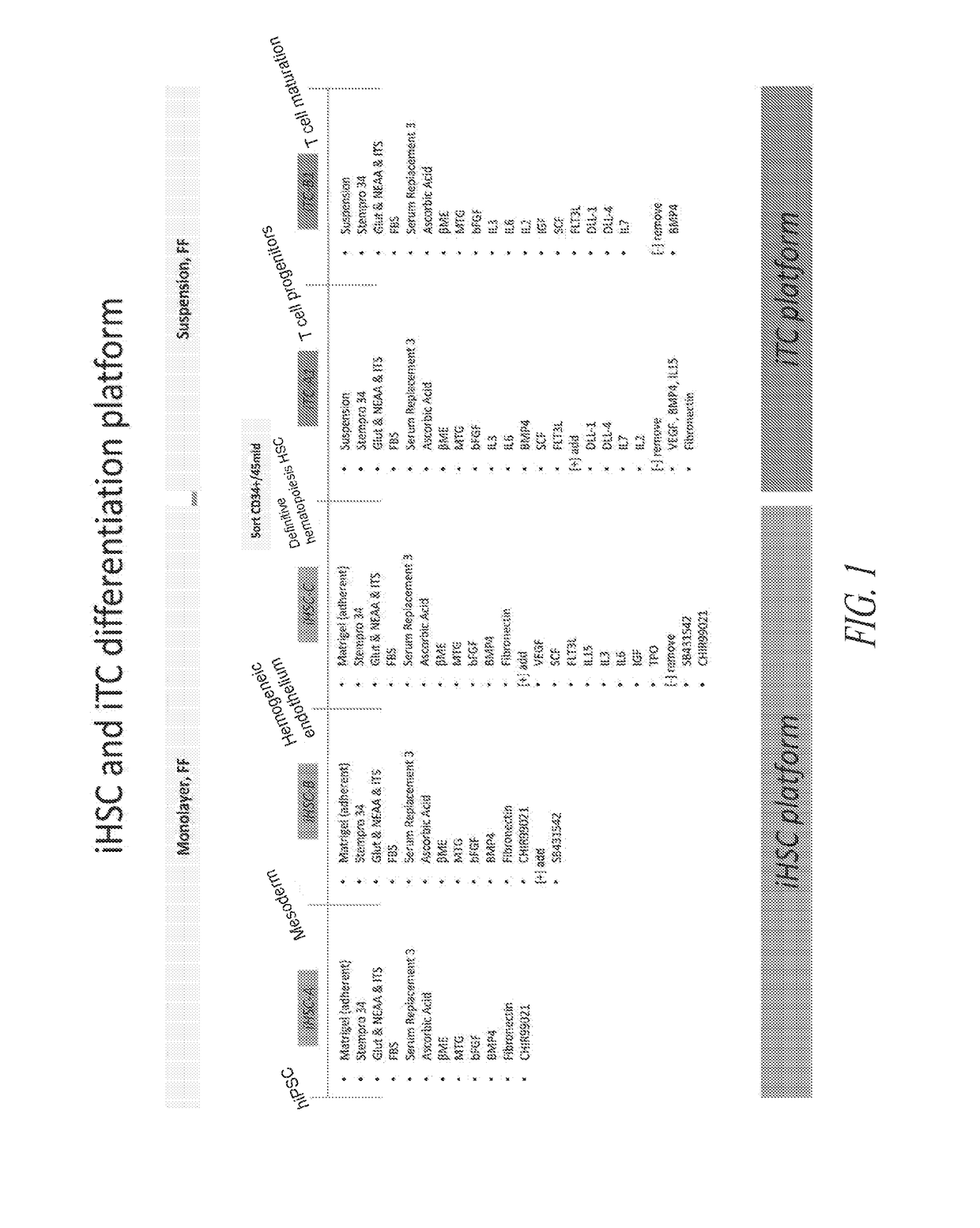
[0316]To initiate differentiation towards hematopoietic cell lineage, naïve hiPSCs were seeded as a monolayer in the maintenance medium and allowed to expand until approximately 25% confluency was reached. At this point, hematopoietic differentiation was initiated by switching the culture medium to iHSC-A (see FIG. 1). As illustrated in FIG. 1, the culture was subsequently switched to iHSC-B, 48hrs post the initiation of differentiation, followed by a switch to iHSC-C on day 4-5 post initiation of differentiation. The attached culture was maintained adherent and not perturbed during the medium changes.
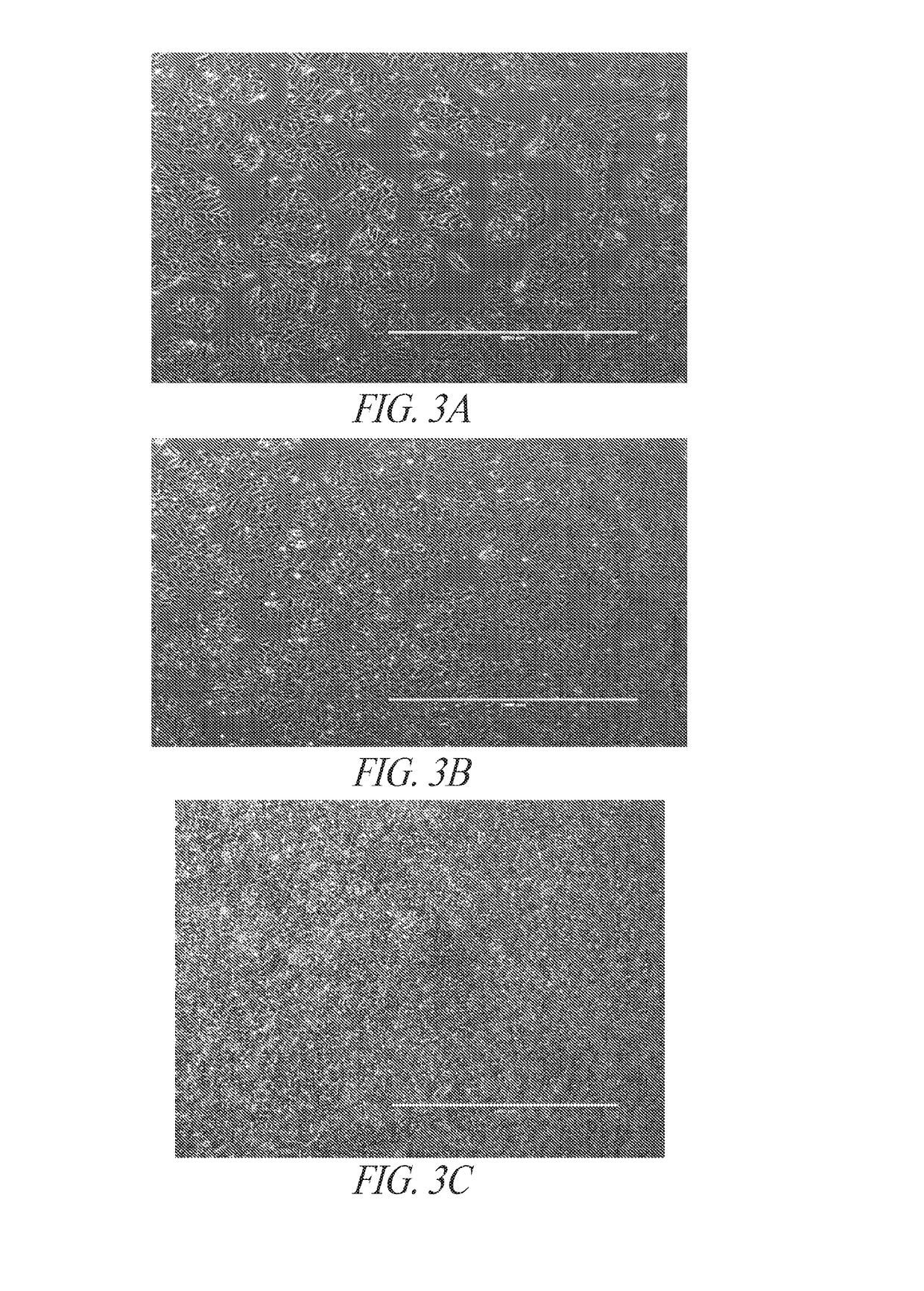
[0317]Early during the differentiation process, directed differentiation was monitored by lineage markers, CD57, NESTIN, SOX17 and BRACHYURY. FIG. 3 illustrates a directed differentiation shift towards the mesoderm lineage and away from ectoderm lineage. Through the subsequent differentiation stages, the hiPSC morphology was give...
example 3
In Vitro Characterization and Testing Following Differentiation Using iHSC Culture Platform
[0318]To determine the expandability and maintenance of the differentiated definitive hematopoietic stem cells, the CD34 sorted or enriched population was transferred into suspended culture supplemented with Stemspan hematopoietic stem cell culture medium (StemCell Technologies, Vancouver, Canada), 1× CC110 supplement (StemCell Technologies), 10 ng / mL bFGF and 5 μM Thiazovivin or 10 μM 27632 ROCK inhibitors (for the first few days in culture to improve survival). The culture was fed with fresh medium every other day and pipetted to break up the aggregates resulting from dividing CD34 positive sorted cells. After several weeks in culture, the scaled suspended culture was assessed and measured through surface marker expression and viable cell number. As demonstrated in FIG. 8, the CD34 sorted population was maintained for 22 days in culture with minimal loss of the CD34 population. To improve vi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com