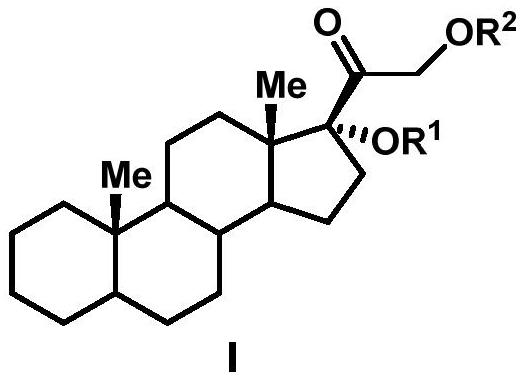
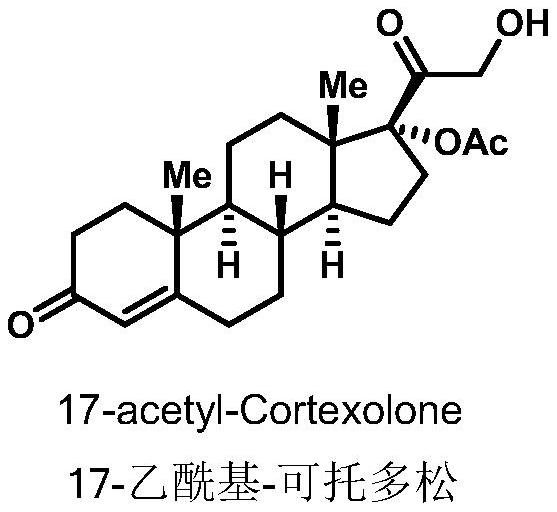
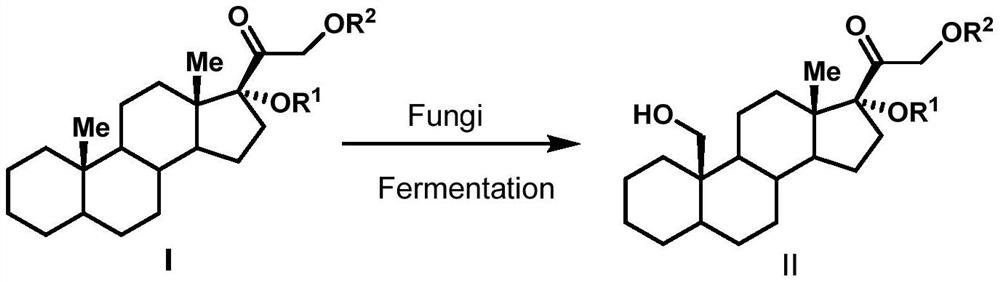
A biotransformation method for the 19-position hydroxylation of a series of steroid compounds
A biotransformation and compound technology, which is applied in the fields of chemistry and biology, can solve the problems of poor selectivity and low yield, and achieve the effects of mild fermentation reaction conditions, high yield, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022]
[0023] Culture condition 1 (solid culture): Inoculate the strain Thanatephorus cucumeris NBRC 6298 (T. cucumeris NBRC6298) on PDA medium (potato 200g / L, glucose 20g / L and agar 15g / L) and activate it in a constant temperature incubator at 30°C to cultivate. After 6-10 days, inoculate the well-growth strains on the plate into a 250mL Erlenmeyer flask containing 100mL yeast liquid medium (glucose 25g / L, yeast extract 20g / L), and grow for about 2-3 days at 30°C and 200rpm 25mg Cortexolone (Cortexolone) was dropped into the thallus culture medium, continued to cultivate and transformed for about 3-4 days until the conversion rate reached the highest, and obtained the compound 19-OH-cortexolone 19-OH-Cortexolone (transformation rate 27%).
[0024] Culture condition 2 (liquid culture): the strain T. cucumeris NBRC 6298 was inoculated on PDA medium for activation culture in a constant temperature incubator at 30°C. After 6-10 days, inoculate the well-growth strains on th...
Embodiment 2
[0027]
[0028]The strain T.cucumeris NBRC 6298 was inoculated on PDA medium for activation culture in a constant temperature incubator at 30°C. After 6-10 days, inoculate the well-growth strains on the plate into a 250mL Erlenmeyer flask containing 100mL yeast liquid medium (glucose 25g / L, yeast extract 20g / L), and grow for about 2-3 days at 30°C and 200rpm , get 5 mL of well-grown bacterium solution and transfer it in a 250 mL Erlenmeyer flask containing 100 mL of fresh yeast liquid medium, continue to cultivate under the same conditions for 2-3 days, and 25 mg of 17-acetyl-cortodopine (17- acetyl-Cortexolone) and ferrous ammonium sulfate were dropped into the thallus culture medium (iron ion final concentration 1.5mmol / L), continued to cultivate and transformed for about 3-4 days until the conversion rate reached the highest, and obtained compound 19-OH-Cortexolone ( 81% conversion).
Embodiment 3
[0030]
[0031] The strain T.cucumeris NBRC 6298 was inoculated on PDA medium for activation culture in a constant temperature incubator at 30°C. After 6-10 days, inoculate the well-growth strains on the plate into a 250mL Erlenmeyer flask containing 100mL yeast liquid medium (glucose 25g / L, yeast extract 20g / L), and grow for about 2-3 days at 30°C and 200rpm , get 5 mL of well-grown bacterium solution and transfer it to a 250 mL Erlenmeyer flask containing 100 mL of fresh yeast liquid medium, continue to cultivate under the same conditions for 2-3 days, and 25 mg of 21-acetyl-cortodopine (21- acetyl-Cortexolone) and ferrous ammonium sulfate were dropped into the thallus culture medium (iron ion final concentration 1.5mmol / L), continued to cultivate and transformed for about 3-4 days until the conversion rate reached the highest, and obtained compound 19-OH-Cortexolone ( Conversion rate 30%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com