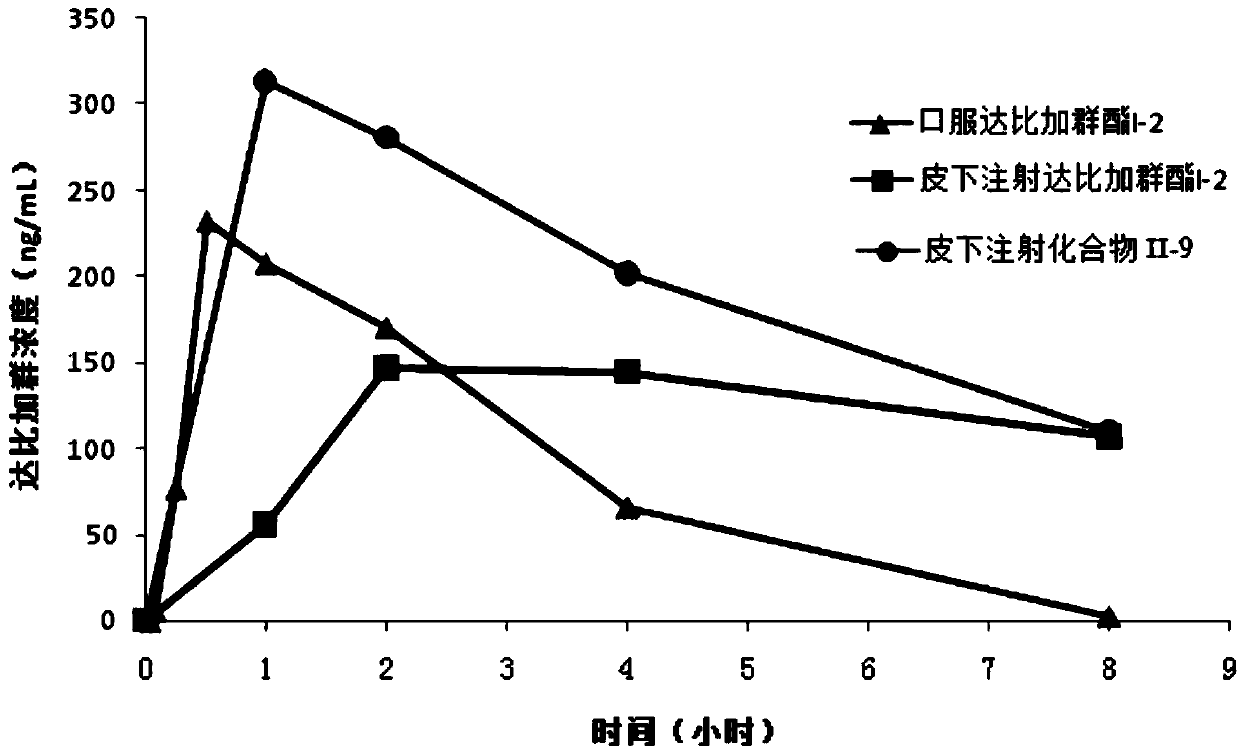
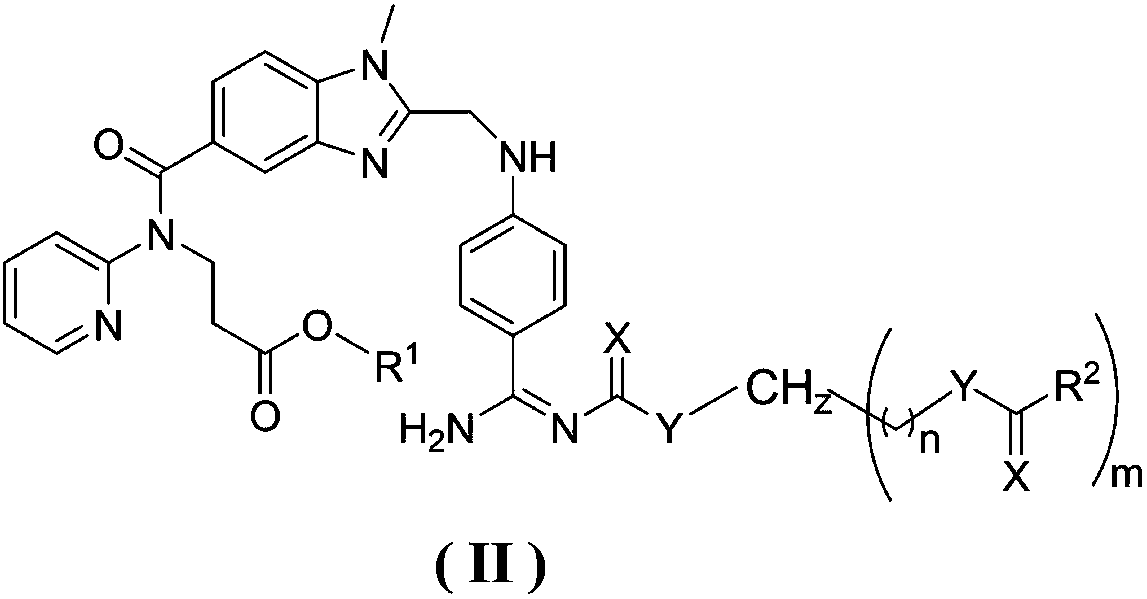
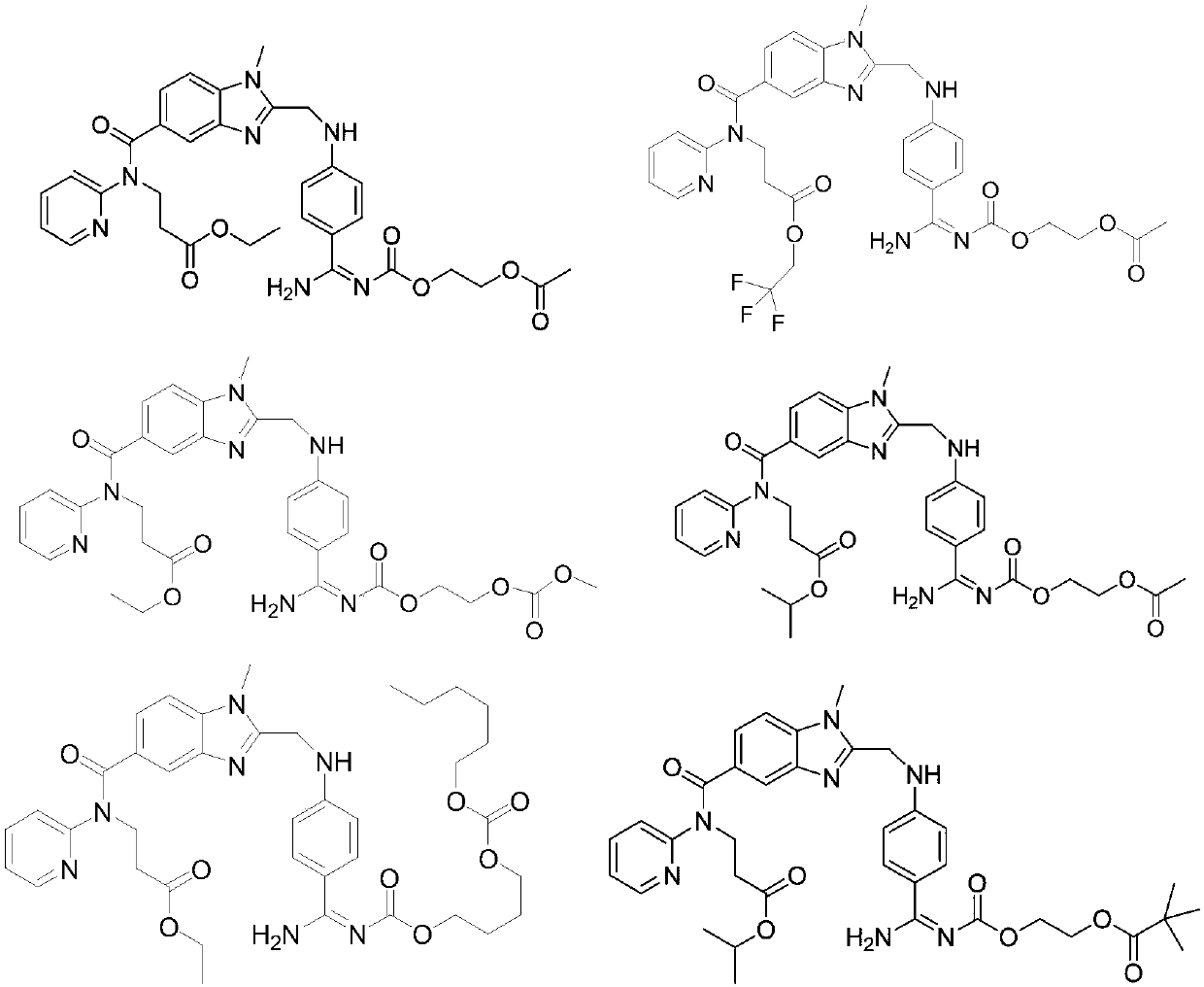
Dabigatran etexilate derivative and pharmaceutical use thereof
A pharmacy and prodrug technology, applied in the field of dabigatran etexilate derivatives and their pharmaceutical uses, can solve the problems of short half-life, easy stimulation of the gastrointestinal tract, strong acidity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0139] The synthesis of embodiment 1 compound II-1
[0140] Compound II-1 was synthesized according to the following reaction formula and experimental steps.
[0141]
[0142] Step 1: Synthesis of Compound 1
[0143] At room temperature, (S)-2-((tert-butoxycarbonyl)amino)-3-phenylpropanoic acid (1.0g, 3.77mmol), ethylene glycol (935mg, 15.1mmol), DCC (1.0g , 4.9mmol) and DMAP (69mg, 0.56mmol) were added to anhydrous dichloromethane (20mL) solvent, after 12 hours of reaction, water (10mL) was added, extracted with dichloromethane (20mL×3), concentrated under reduced pressure The organic phase was removed, and the residue was purified by silica gel column chromatography (petroleum ether / ethyl acetate=10 / 1 to 3 / 1) to obtain compound 1 (900 mg) as a white solid.
[0144] 1 H NMR (300MHz, DMSO-d 6 ):δ7.27-7.16(m,5H),4.19-4.14(m,1H),4.03-3.98(m,2H),3.52(s,2H),3.05-2.80(m,2H),1.98(s ,1H), 1.34(s,9H).
[0145] Step 2: Synthesis of Compound 2
[0146] At room temperature, CDI...
Embodiment 2
[0153] Embodiment 2: the synthesis of compound II-2
[0154] Compound II-2 was synthesized according to the following reaction formula and experimental steps.
[0155]
[0156] Step 1: Synthesis of compound 3
[0157] Pyridine-3-carboxylic acid chloride (1.80 g, 12.5 mmol) and DIPEA (2.20 g, 16.3 mmol) were added to glycerol (500 mg, 5.43 mmol) in DMF (5 mL) at room temperature and stirred for 16 hours. It was concentrated under reduced pressure, and the residue was purified by silica gel column chromatography, eluting with dichloromethane / methanol=10:1, to obtain compound 3 (510 mg) as a yellow solid.
[0158] 1 H NMR (300MHz, CDCl 3 ): δ9.12(s, 2H), 8.16(d, J=3.6Hz, 2H), 8.33(t, J=4.5Hz, 2H), 7.56(dd, J=7.8, 4.8Hz, 2H), 4.41 -4.39(m,4H),4.25-4.23(m,1H).
[0159] Step 2: Synthesis of compound 4
[0160] At room temperature, CDI (70 mg, 0.437 mmol) was added to a solution of compound 3 (120 mg, 0.40 mmol) in DMF (20 mL), and stirred at 50° C. for 16 hours. After cool...
Embodiment 3
[0165] Embodiment 3: the synthesis of compound II-3
[0166] Compound II-3 was synthesized according to the following reaction formula and experimental steps.
[0167]
[0168] At room temperature, 2-hydroxyethyl-acetate (0.77g, 3.73mmol) was added to DMF (5mL) solution, CDI (0.3g, 1.87mmol) was added, and DIPEA (0.14g, 1.12 mmol) and compound I-4 (0.2g, 0.37mmol), stirred for 12 hours. Concentrated under reduced pressure and purified by HPLC to obtain compound II-3 (120 mg) as a white solid.
[0169] 1 H NMR(400MHz,DMSO-d6):δ9.17(br s,1H),8.72(br s,1H),8.39(s,1H),7.81(d,J=8.8Hz,2H),7.56-7.52 (m,1H),7.47(s,1H),7.40(d,J=8.4Hz,1H),7.16-7.10(m,2H),7.02-6.99(m,1H),6.88(d,J=7.6 Hz,1H),6.76(d,J=8.4Hz,2H),4.59(d,2H),4.31-4.17(m,6H),3.96(q,2H),3.77(s,3H),2.66(t , J=7.2Hz, 2H), 1.97(s, 6H), 1.12(t, J=7.2Hz, 3H).
[0170] LCMS: Rt=3.194min, [M+H] + = 630.3.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap