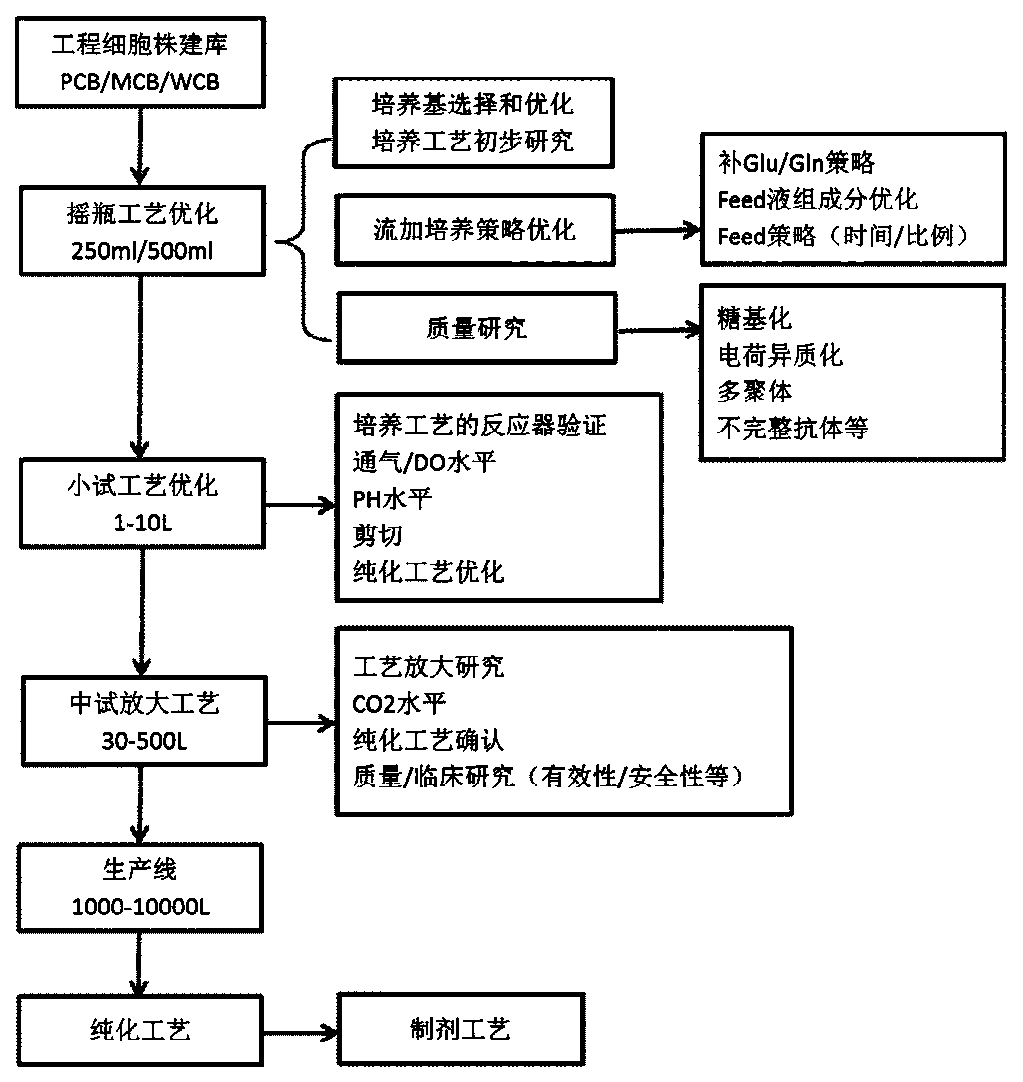
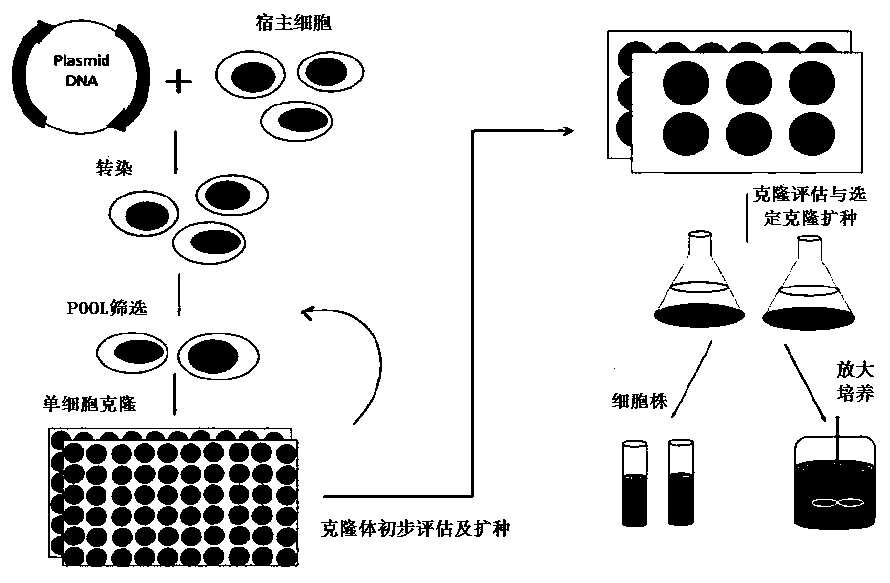
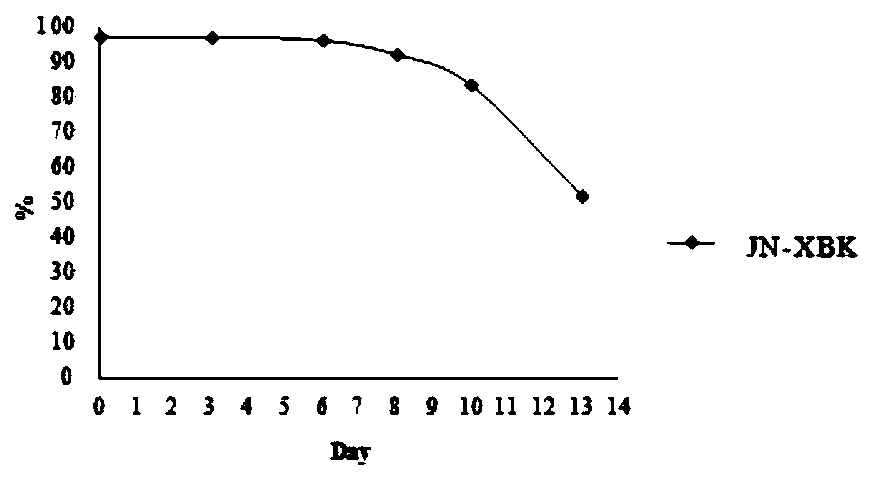
Cell strain constructing method for preparing anti-EGFR completely-humanized monoclonal antibody
A technique of monoclonal antibody and construction method, which is applied in the field of cell line construction for the preparation of fully humanized anti-EGFR monoclonal antibody, which can solve the problems of limiting the research and development and industrialization of biosimilar drugs, and the impact of final product expression or activity. Achieve the effects of mild reaction conditions, easy industrialization, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Transfection experiment:
[0050] Experimental Materials:
[0051] Host cell: DG44 (Chinese hamster ovary cell dihydrofolate reductase-deficient strain CHO / DHFR-, ATCC)
[0052] Transfection reagent: Freestyle Max Reagent (Invitrogen 16447-100)
[0053] OPTI PRO SFM (GIBCO 12309-019)
[0054] Linearized plasmid: pCHO1.0invitrogen
[0055] DG44 complete medium: CD DG44 (Invitrogen 12610-010) contains
[0056] 4mM GlutaMAX-I (Invitrogen 35050-061)
[0057] 18ml / L PLURONIC F68(PF68)(Invitrogen 24040-032)
[0058] Consumables and related instruments
[0059] CORNING 125ml Erlenmeyer Flask, Item No. 431143;
[0060] Shaker INFORS HT Model: Multitron II
[0061] Parameter setting: 110rpm, 36.5℃, 6%CO 2 , humidity 75%;
[0062] Cell Counter BECKMAN COULTER Model: VI-CELL XR
[0063] Biological Safety Cabinet Shanghai Shangjing Model: BSC-ⅡA2
[0064] Transfection experimental steps:
[0065] 1. 24h before transfection, the DG44 cells for standby transfection were ...
Embodiment 2
[0136] Equipment and Consumables
[0137] Constant temperature water bath Julabo Model: TW20
[0138] Biological Safety Cabinet Shanghai Net Model: BSC-Ⅱ A2
[0139] CO2 static incubator THERMO SCIENTIFIC model: HERA cell240i
[0140] Cell Counter BECKMAN COULTER Model: VI-CELL XR
[0141] Centrifuge EPPENDORF Model: Centrifuge 5810R
[0142] ClonePix GENETIX Model: Clonepix FL
[0143] 96-well plate (greiner bio-one CELLSTAR, Cat. No. 655185)
[0144] 24-well plate (greiner bio-one CELLSTAR, Cat. No. 662102)
[0145] 6-well plate (greiner bio-one CELLSTAR, Cat. No. 657185)
[0146] Shaker KUHNER Model: Climo-Shaker ISF1-XC
[0147] Parameter setting: 225rpm, 36.5°C, 6% CO2, 85% humidity.
[0148] Shaker TPP TUBESPIN BIOREACTOR 50, Item No.: 87050
[0149] The culture medium and reagents are shown in Table 5:
[0150] table 5
[0151] Medium name brand article number SFM4 ADCF Hyclone SH3A2156.01 Glutamine Sigma G8540 MTX Sigma ...
Embodiment 3
[0207] The invention selects the stable cell line JK-I with high expression of Vectibix through POOL construction, batch feeding experiment screening and plating, clone picking, HTRF, clone amplification, and clone batch feeding experiment. Through process optimization, the expression amount reached 2.05g / L, and the product SEC, pI-cIEF, deglycosylated molecular weight, and cell-based antibody activity were significantly improved. Example 3 Cell Line Stability Test
[0208] The genetic stability of cell lines is the basis for subsequent stable production processes and product quality. The genetic stability of the cells in the PCB (Primary Cell Bank), MCB (Master Cell Bank), and WCB (Work Cell Bank) three cell banks in the project was studied by molecular biology methods, including heavy chain (HC) and light Stranded (LC) DNA sequence stability and copy number stability. The project plans to carry out cell line stability research for no less than 12 weeks, including cell grow...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com