Diterpenoid glycoside compound in glechoma longituba and extraction and separation method thereof
A compound and composition technology, applied in the field of medicine, can solve problems such as the lack of diterpene and its glycoside components, reports, complex chemical components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
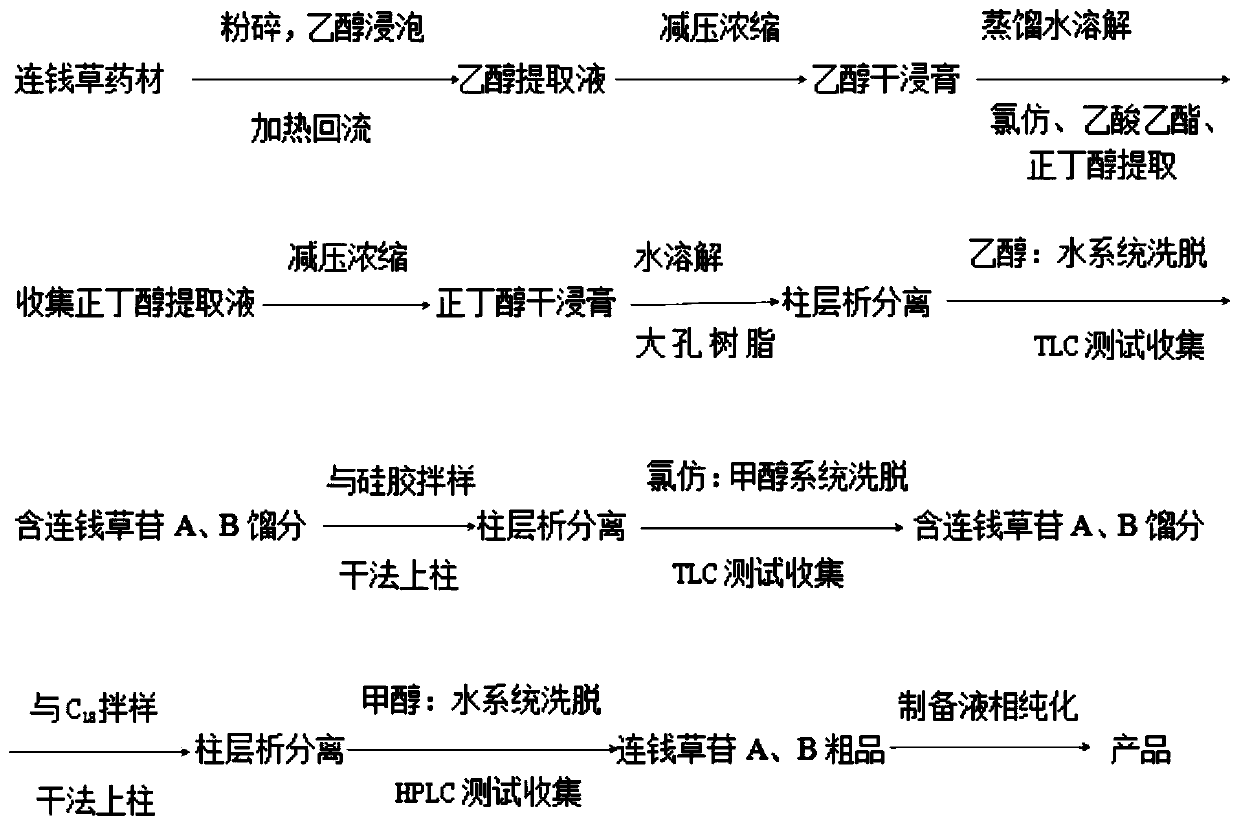
[0063] Example 1: Extraction and separation of the diterpenoid glycosides diclairoside A and B in Dichrysalis:
[0064] Lianqian herbal medicine was collected from Huoshan County, Lu'an City, Anhui Province on June 12, 2016. It was identified as Glechoma longituba (Nakai) Kupr, a plant of the Labiatae family, by Wan Linchun, deputy director of the Jiangxi Provincial Institute of Drug Inspection and Testing. of whole grass. The specimen is kept in the Jiangxi Provincial Drug Inspection and Testing Research Specimen Laboratory (specimen number JXSYJY2016012)
[0065] The extraction and separation steps of the diterpene glycosides Dilimoside A and B are as follows:
[0066] (1) Heating and reflux extraction with ethanol: Dried Dilimocarpis in the shade and then pulverized, 50.0Kg of the pulverized Herba Lisiana, heated and refluxed with 75% ethanol for 3 times, filtered to obtain a 75% ethanol extract;
[0067] (2) Concentrating 75% ethanol extract: 75% ethanol extract (EYELA S...
Embodiment 2
[0072] Example 2: Structural Identification of Diterpenoid Glycosides Dicarinaside A and B:
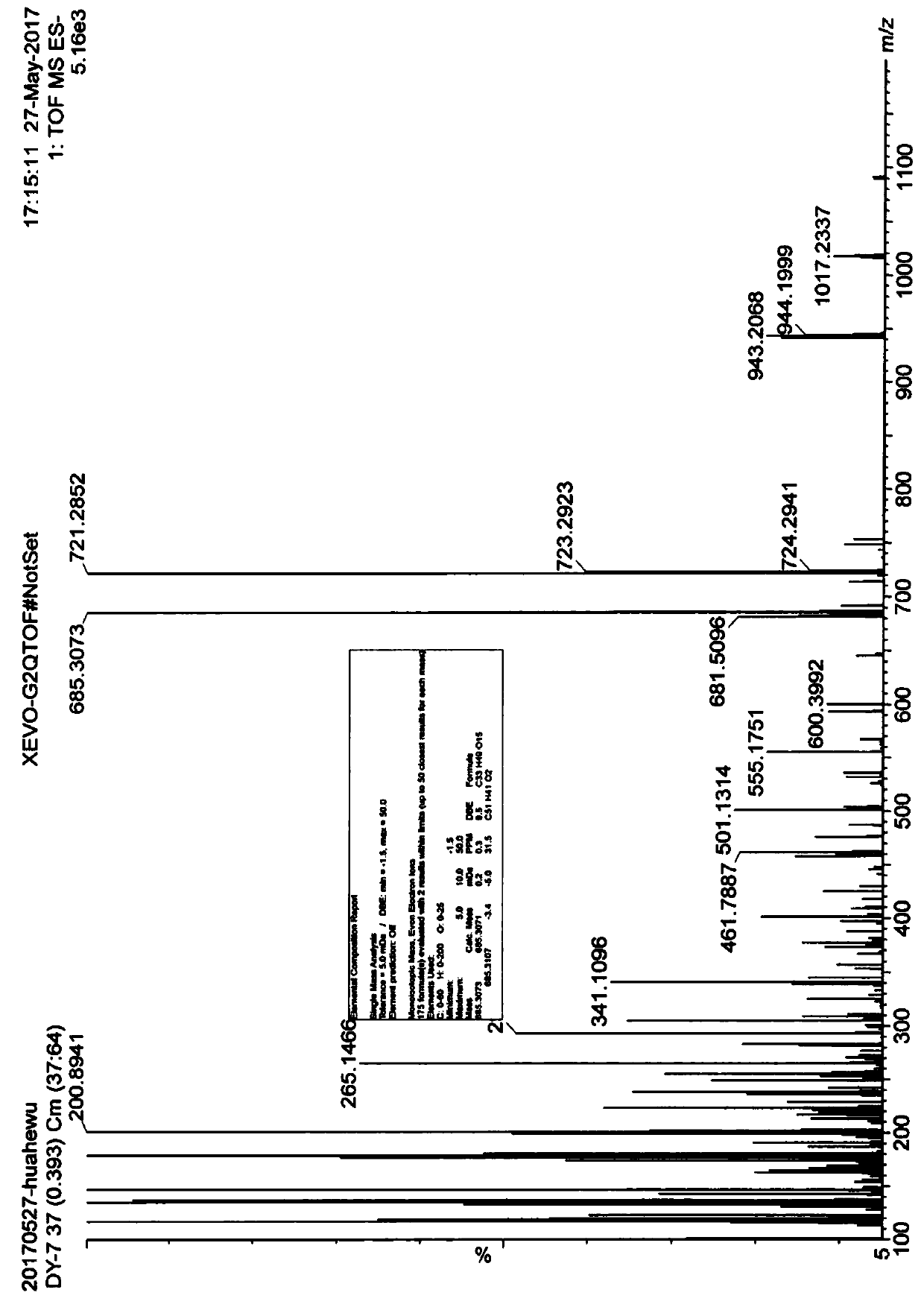
[0073] Two new diterpene glycosides were identified with molecular formulas of C 33 h 50 o 15 and C 33 h 48 o 15 , named as liancaoside A and liancaoside B, and its chemical structural formula is:
[0074]
[0075] Table 1 is the NMR data (Varian UNITY INOVA 600 type superconducting NMR instrument (Varian Co., Ltd., U.S.)) of these two new diterpene glycoside compounds: 1 H-NMR with 13 C-NMR on CD 3 OD.
[0076]Table 1: NMR data of the diterpene glycosides diluciside A and diluciside B of the present invention.
[0077]
[0078]
[0079]
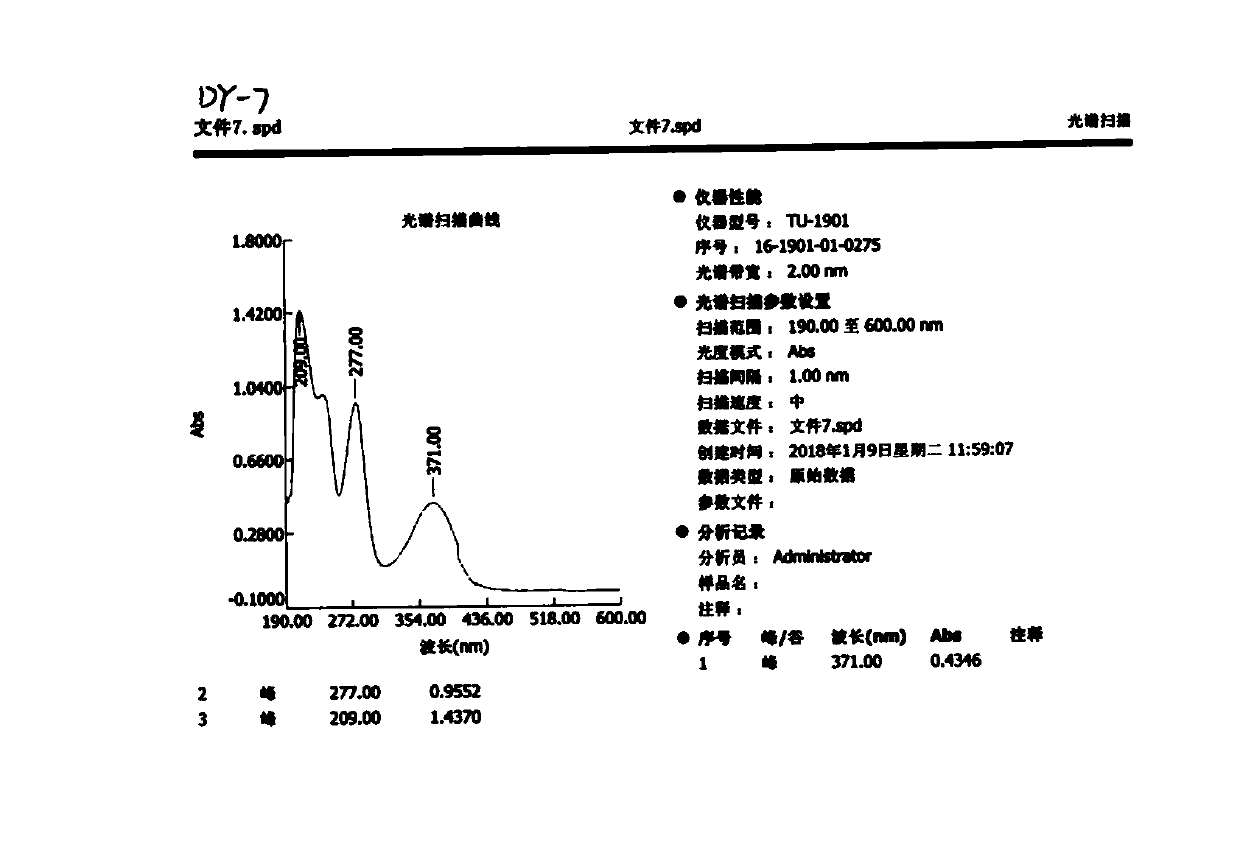
[0080] Please refer to Figure 2-8 .
[0081] Limoside A: Pale yellow powder, soluble in methanol. The melting point is 225-227°C measured by RY-IG melting point analyzer (Tianjin Tianguang Optical Instrument Co., Ltd., China), and measured by Perkin-Elmer 341 polarimeter (PERKIN ELMER Co., Ltd., USA)[α] 20 D -4.0 (c 0.025,...
Embodiment 3
[0090] Example 3: In vitro anti-tumor activity test of diterpenoid glycosides dicarinaside A and B:
[0091] Tumor cell growth inhibition rate (%)=(measured value of test well / measured value of control well)×100%
[0092] Test principle: MTT method: There is a dehydrogenase related to NAPP (nicotinamide adenine dinucleotide phosphate, coenzyme II) in the mitochondria of living cells, and succinate dehydrogenase can make exogenous yellow thiazolium blue MTT ( 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) is reduced to water-insoluble blue-violet crystalline formazan (Formazan) and deposited in cells, dead cells During this enzyme disappears, MTT is not reduced. After dissolving formazan with dimethyl sulfoxide (DMSO), the absorbance can be detected at 570nm and 630nm with a microplate reader, and the optical density value is proportional to the number of viable cells.
[0093] The cell lines used are: BGC-823 (human gastric cancer cells), Bel (human liver canc...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap