Nucleoside derivative or salt thereof, polynucleotide synthesis reagent, polynucleotide production method, polynucleotide, and binding nucleic acid molecule production method
A technology of nucleoside derivatives and polynucleotides, which is applied in the field of nucleoside derivatives or their salts, and can solve problems such as inability to obtain binding nucleic acid molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] IA8 was prepared by the following Synthesis Example.
[0089] Electrospray ionization mass spectrometry (ESI-MS) was performed using a mass spectrometer (API2000, supplier: Applied Biosystems) in positive or negative ion mode. Obtained using a nuclear magnetic resonance apparatus (JNM-ECS400, manufactured by JEOL) 1 H NMR spectrum. Chemical shifts are expressed relative to the internal standard tetramethylsilane (Me 4 δ (ppm) of Si). Ion exchange chromatography was performed using a chromatography system (ECONO system, manufactured by Bio-Rad). In ion exchange chromatography, a glass column packed with diethylaminoethyl (DEAE) A-25-Sephadex is used (manufactured by Amershambiosciences).
[0090] (Synthesis Example 1) Synthesis of TB4
[0091]
[0092] 2-Bromoethylamine hydrobromide (501 mg, 2.28 mmol) was weighed and suspended in 1,4-dioxane (5 ml), and the mixture thus obtained was stirred under ice bath for 30 minutes. To the mixture thus obtained was added...
Embodiment 2
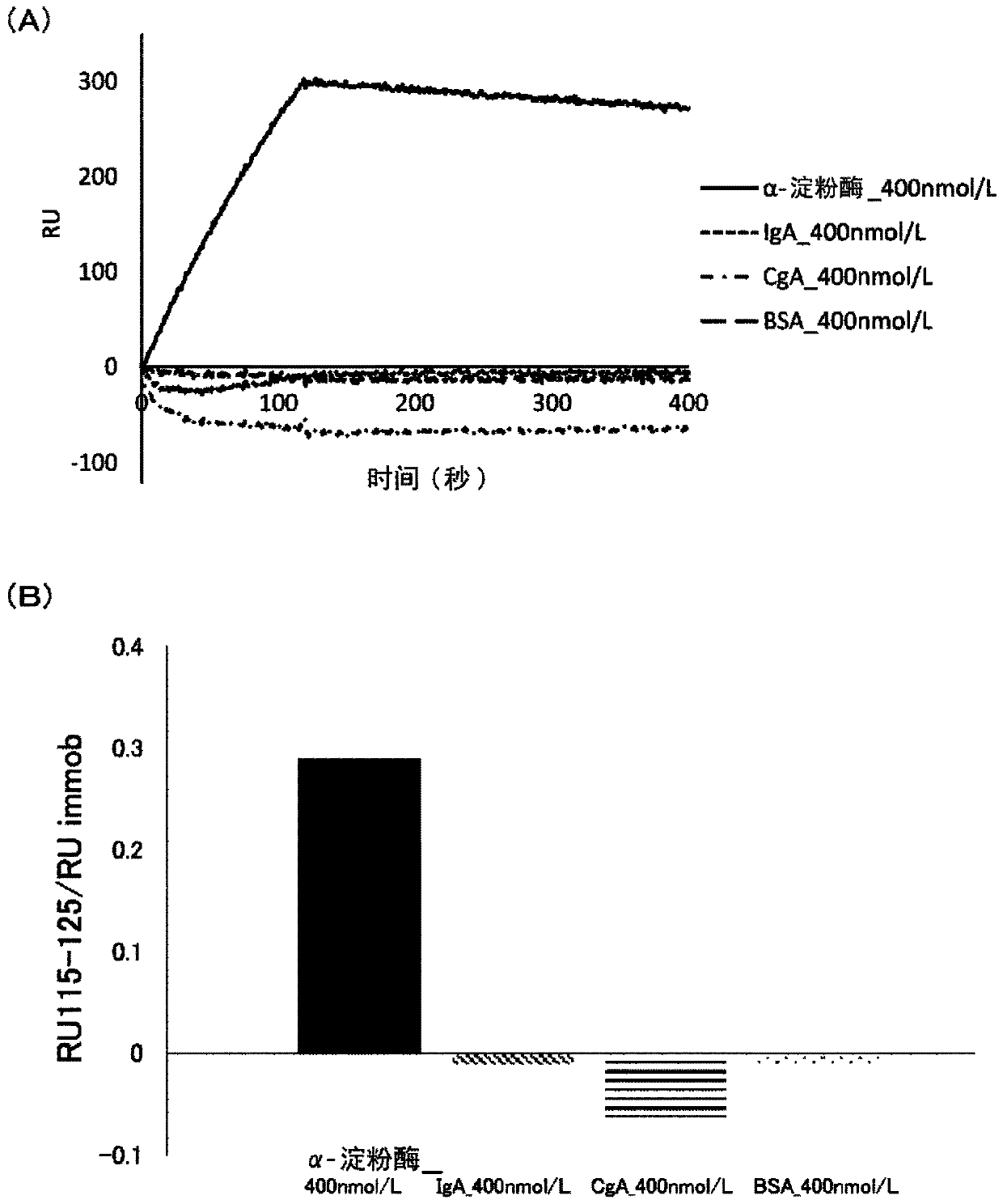
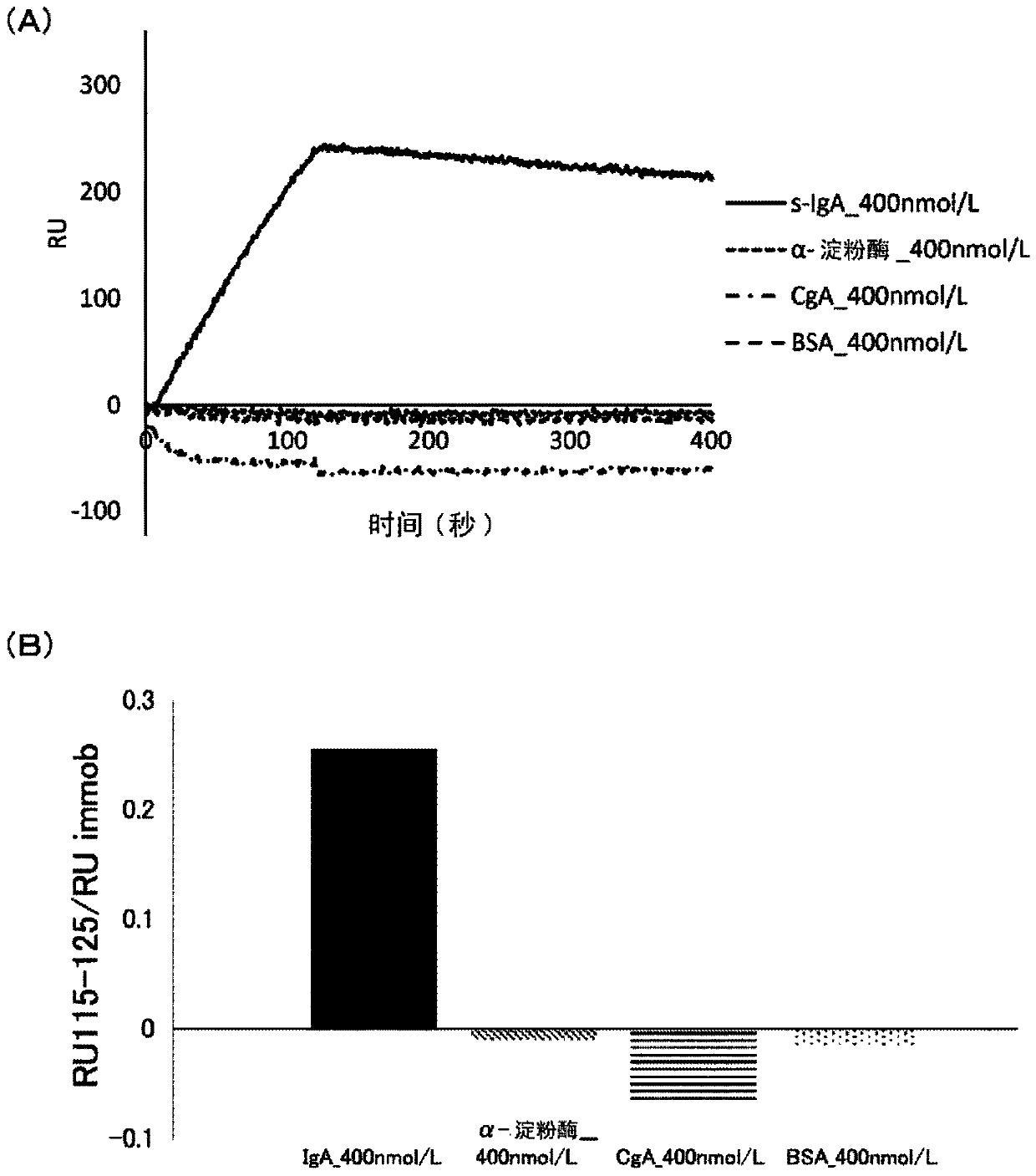
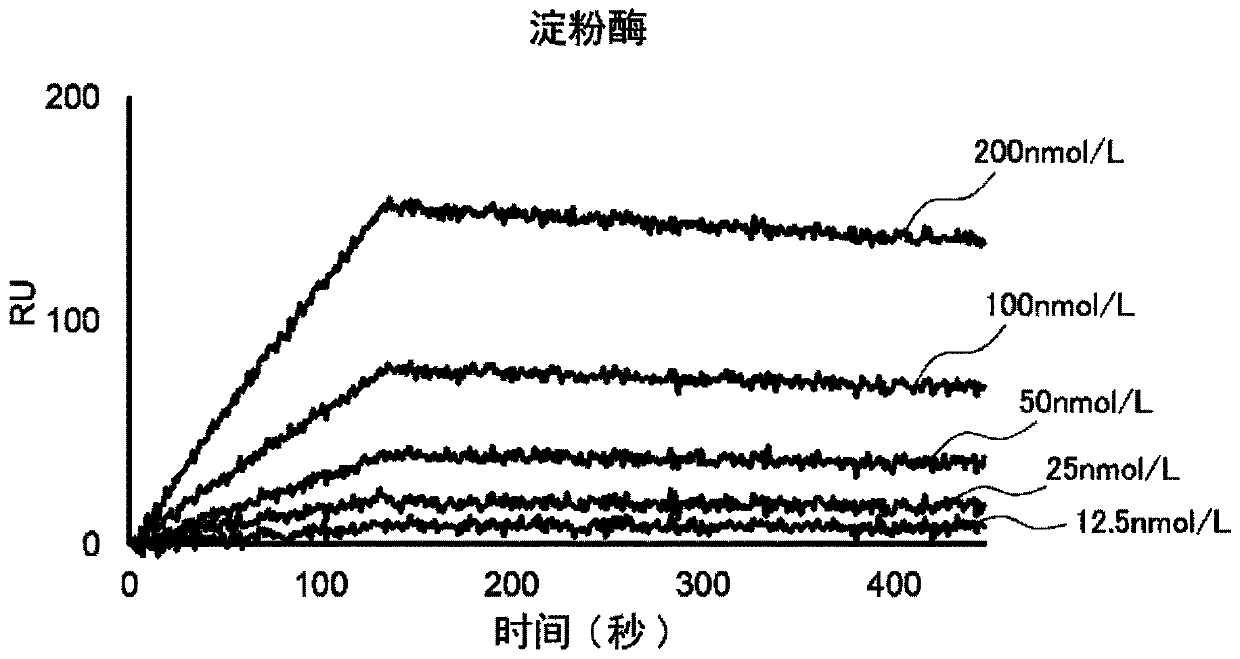
[0147] This example examines whether sIgA-binding and amylase-binding nucleic acid molecules can be obtained using IA8.
[0148] (1) Binding to nucleic acid molecules
[0149] Binding nucleic acid molecules binding to the respective targets were obtained by the SELEX method except that, in addition to deoxyribonucleotides containing adenine, guanine and cytosine (dATP, dGTP and dCTP, respectively), IA8 was used as the deoxyribose Nucleotides to prepare candidate polynucleotides. Specifically, a binding nucleic acid molecule is obtained in the following manner. According to the protocol attached to the product, sIgA (product of MP Biomedicals, LLC-Cappel) or human salivary amylase (manufactured by LeeBioSolutions Corporation) was bound as a target to beads (Dynabeads MyOne Carboxylic Acid, manufactured by Invitrogen). After binding the target, use selection buffer (SB buffer: 40mmol / L HEPES, 125mmol / L NaCl, 5mmol / L KCl, 1mmol / L MgCl 2 , 0.01%Tween pH 7.5) to wash the beads...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap